Thailand's Psychotropic Substances Act is a law designed to regulate certain mind-altering drugs. According to the Office of the Narcotics Control Board, "The Act directly resulted from the Convention on Psychotropic Substances 1971 of which Thailand is a party." The Act divides psychotropic drugs into four Schedules. Offenses involving Schedule I and II drugs carry heavier penalties than those involving Schedule III and IV drugs. Note that this statute does not regulate most opioids, cocaine, or some amphetamines. The vast majority of narcotic painkillers, along with cocaine and most amphetamines are regulated under the Narcotics Act.
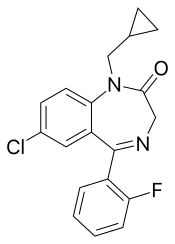
Fludiazepam, marketed under the brand name Erispan (エリスパン) is a potent benzodiazepine and 2ʹ-fluoro derivative of diazepam, originally developed by Hoffman-La Roche in the 1960s. It is marketed in Japan and Taiwan. It exerts its pharmacological properties via enhancement of GABAergic inhibition. Fludiazepam has 4 times more binding affinity for benzodiazepine receptors than diazepam. It possesses anxiolytic, anticonvulsant, sedative, hypnotic and skeletal muscle relaxant properties. Fludiazepam has been used recreationally.

Ethyl loflazepate is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses. Its elimination half-life is 51–103 hours. Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound. Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate. The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate. Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment. The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children. Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity. High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.

Fludroxycortide, also known as flurandrenolide (USAN) and flurandrenolone, is a synthetic topical corticosteroid and is used as an anti-inflammatory treatment for use on skin irritations. Trade names include Haelan and Cordran.

Oxatomide, sold under the brand name Tinset among others, is a first-generation antihistamine of the diphenylmethylpiperazine family which is marketed in Europe, Japan, and a number of other countries. It was discovered at Janssen Pharmaceutica in 1975. Oxatomide lacks any anticholinergic effects. In addition to its H1 receptor antagonism, it also possesses antiserotonergic activity similarly to hydroxyzine.
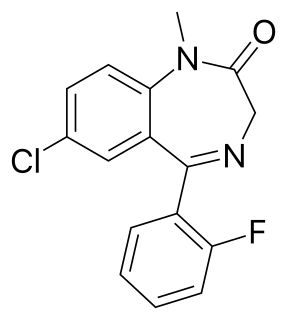
Flutazolam is a drug which is a benzodiazepine derivative. It was invented in Japan, and this is the main country in which it has been used medically. It has sedative, muscle relaxant, anticonvulsant, and anxiolytic effects similar to those produced by other benzodiazepine derivatives, and though it is around the same potency as diazepam, it produces a more marked sedation and impaired coordination. It is indicated for the treatment of insomnia. Its major active metabolite is n-desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam. While flutazolam has a very short half-life of only 3.5 hours, n-desalkylflurazepam has a long half-life of between 47-100 hours.

Rilmazafone is a water-soluble prodrug developed in Japan. Once metabolized, rilmazafone is converted into several benzodiazepine metabolites that have sedative and hypnotic effects. These metabolites induce impairment of motor function and have hypnotic properties.
Lafutidine (INN) is a second generation histamine H2 receptor antagonist having multimodal mechanism of action and used to treat gastrointestinal disorders. It is marketed in South Korea, Japan and India.
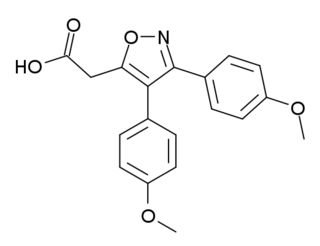
Mofezolac (INN), sold under the name Disopain in Japan, is a nonsteroidal anti-inflammatory drug (NSAID) used for its analgesic and anti-inflammatory actions. It is often prescribed for rheumatoid arthritis, lower back pain, frozen shoulder, and pain management after surgery or trauma. It is also being investigated for potential use in the treatment of neuroinflammation.
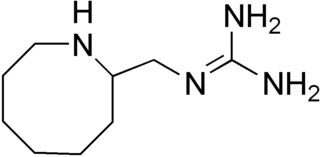
Guanazodine is a hypotensive sympatholytic drug.

Mosapramine (Cremin) is an atypical antipsychotic used in Japan for the treatment of schizophrenia. It is a potent dopamine antagonist with high affinity to the D2, D3, and D4 receptors, and with moderate affinity for the 5-HT2 receptors.

Phellodendrine is an alkaloid isolated originally from Phellodendron amurense (Rutaceae).
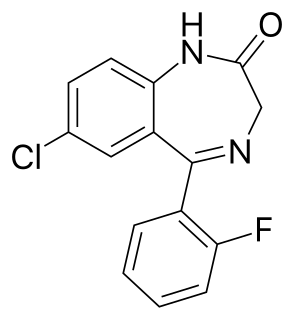
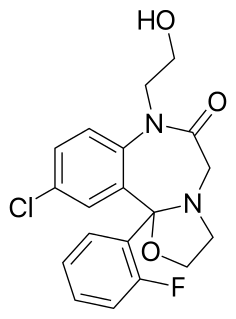
N-Desalkylflurazepam is a benzodiazepine analog and an active metabolite of several other benzodiazepine drugs including flurazepam, flutoprazepam, fludiazepam, midazolam, flutazolam, quazepam, and ethyl loflazepate. It is long-acting, prone to accumulation, and binds unselectively to the various benzodiazepine receptor subtypes. It has been sold as a designer drug from 2016 onward.
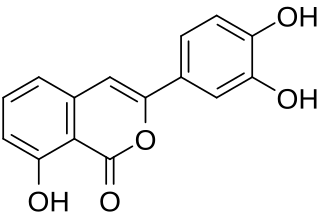
Thunberginol A is an isocoumarin found in Hydrangea macrophylla and the herbal preparation hydrangeae dulcis folium which is produced from its leaves.

Teniloxazine, also known as sufoxazine and sulfoxazine, is a drug which is marketed in Japan. Though initially investigated as a neuroprotective and nootropic agent for the treatment of cerebrovascular insufficiency in the 1980s, it was ultimately developed and approved as an antidepressant instead. It acts as a potent norepinephrine reuptake inhibitor, with fair selectivity over the serotonin and dopamine transporters, and also behaves as an antagonist of the 5-HT2A receptor.
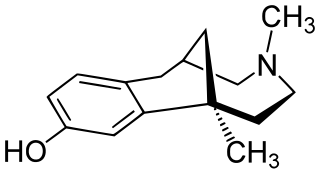
Eptazocine (Sedapain) is an opioid analgesic which was introduced in Japan by Morishita in 1987. It acts as a mixed κ-opioid receptor agonist and μ-opioid receptor antagonist.
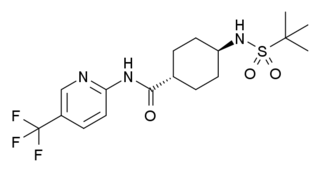
Velneperit (S-2367) is a drug developed by Shionogi, which acts as a potent and selective antagonist for the Neuropeptide Y receptor Y5. It has anorectic effects and was developed as a possible treatment for obesity, but was discontinued from further development after disappointing results in Phase II clinical trials. However it was still considered a successful proof of concept of the potential of Y5 receptor antagonists as possible anti-obesity agents in future.
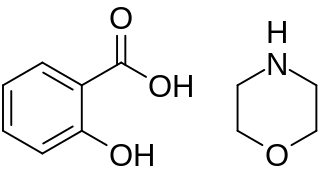
Morpholine salicylate is a nonsteroidal anti-inflammatory drug.
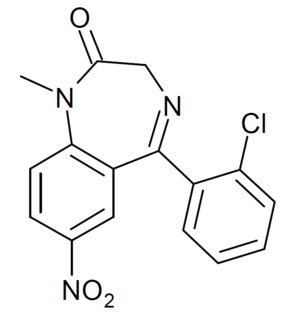
Ro05-4082 is a benzodiazepine derivative developed in the 1970s. It has sedative and hypnotic properties, and has around the same potency as clonazepam itself. It was never introduced into clinical use. It is a structural isomer of meclonazepam (3-methylclonazepam), and similarly has been sold as a designer drug, first being identified in Sweden in 2017.