
Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.

Procaine is a local anesthetic drug of the amino ester group. It is most commonly used in dental procedures to numb the area around a tooth and is also used to reduce the pain of intramuscular injection of penicillin. Owing to the ubiquity of the trade name Novocain or Novocaine, in some regions, procaine is referred to generically as novocaine. It acts mainly as a sodium channel blocker. Today it is used therapeutically in some countries due to its sympatholytic, anti-inflammatory, perfusion-enhancing, and mood-enhancing effects.

Sevoflurane, sold under the brand name Sevorane, among others, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desflurane, it is the volatile anesthetic with the fastest onset. While its offset may be faster than agents other than desflurane in a few circumstances, its offset is more often similar to that of the much older agent isoflurane. While sevoflurane is only half as soluble as isoflurane in blood, the tissue blood partition coefficients of isoflurane and sevoflurane are quite similar. For example, in the muscle group: isoflurane 2.62 vs. sevoflurane 2.57. In the fat group: isoflurane 52 vs. sevoflurane 50. As a result, the longer the case, the more similar will be the emergence times for sevoflurane and isoflurane.
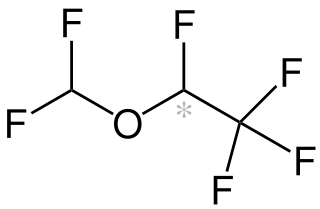
Desflurane is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane, and isoflurane, it is a racemic mixture of (R) and (S) optical isomers (enantiomers). Together with sevoflurane, it is gradually replacing isoflurane for human use, except in economically undeveloped areas, where its high cost precludes its use. It has the most rapid onset and offset of the volatile anesthetic drugs used for general anesthesia due to its low solubility in blood.

Enflurane is a halogenated ether. Developed by Ross Terrell in 1963, it was first used clinically in 1966. It was increasingly used for inhalational anesthesia during the 1970s and 1980s but is no longer in common use.

Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf. It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil. Eugenol has a pleasant, spicy, clove-like scent. The name is derived from Eugenia caryophyllata, the former Linnean nomenclature term for cloves. The currently accepted name is Syzygium aromaticum.

Oil of clove, also known as clove oil, is an essential oil extracted from the clove plant, Syzygium aromaticum. Clove oil is commonly used in aromatherapy and for flavoring food and some medicines. Madagascar and Indonesia are the main producers of clove oil.

Minaxolone (CCI-12923) is a neuroactive steroid which was developed as a general anesthetic but was withdrawn before registration due to toxicity seen with long-term administration in rats, and hence was never marketed. It is a positive allosteric modulator of the GABAA receptor, as well as, less potently, a positive allosteric modulator of the glycine receptor.

Alfaxalone, also known as alphaxalone or alphaxolone and sold under the brand name Alfaxan, is a neuroactive steroid and general anesthetic which is used currently in veterinary practice as an induction agent for anesthesia and as an injectable anesthetic. Though it is more expensive than other induction agents, it often preferred due to the lack of depressive effects on the cardiovascular system. The most common side effect seen in current veterinary practice is respiratory depression when Alfaxan is administered concurrently with other sedative and anesthetic drugs; when premedications aren't given, veterinary patients also become agitated and hypersensitive when waking up.

tert-Amyl alcohol (TAA) or 2-methylbutan-2-ol (2M2B), is a branched pentanol.
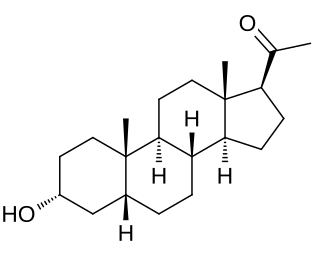
Pregnanolone, also known as eltanolone, is an endogenous inhibitory neurosteroid which is produced in the body from progesterone. It is closely related to allopregnanolone, which has similar properties.
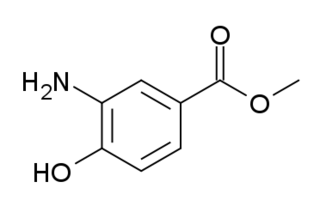
Orthocaine is a local anesthetic. Developed in the 1890s, it was found to be of limited use due to its low solubility in water, but it has been used in powdered form to dust onto painful wounds.

Hydroxydione, as hydroxydione sodium succinate, also known as 21-Hydroxy-5β-pregnane-3,20-dione, is a neuroactive steroid which was formerly used as a general anesthetic, but was discontinued due to incidence of thrombophlebitis in patients. It was introduced in 1957, and was the first neuroactive steroid general anesthetic to be introduced for clinical use, an event which was shortly preceded by the observation in 1954 of the sedative properties of progesterone in mice.

Alfadolone (INN), or alphadolone is a neuroactive steroid and general anesthetic. Along with alfaxolone, as alfadolone acetate, it is one of the components of the anesthetic drug mixture althesin.

Butidrine (INN), or butedrine or butydrine, also known as hydrobutamine or idrobutamine, is a beta blocker related to pronethalol and propranolol that was developed in the 1960s. Similarly to certain other beta blockers, butidrine also possesses local anesthetic properties.

Fluroxene, or 2,2,2-trifluoroethyl vinyl ether, is a volatile, inhalational anesthetic, and was the first halogenated hydrocarbon anesthetic to be introduced. It was synthesized in 1951, and was introduced for clinical use in 1954, but was voluntarily withdrawn from the market in 1974 due to its potential flammability and accumulating evidence that it could cause organ toxicity. In any case, prior to being discontinued, it had largely been superseded by halothane. Fluroxene is metabolized to 2,2,2-trifluoroethanol, a compound responsible for some of the toxicity seen with fluroxene use.

Norketamine, or N-desmethylketamine, is the major active metabolite of ketamine, which is formed mainly by CYP3A4. Similarly to ketamine, norketamine acts as a noncompetitive NMDA receptor antagonist, but is about 3–5 times less potent as an anesthetic in comparison. Also, similarly again to ketamine, norketamine binds to the μ- and κ-opioid receptors. Relative to ketamine, norketamine is much more potent as an antagonist of the α7-nicotinic acetylcholine receptor, and produces rapid antidepressant effects in animal models which have been reported to correlate with its activity at this receptor. However, norketamine is about 1/5 as potent as ketamine as an antidepressant in mice as per the forced swim test, and this seems also to be in accordance with its 3–5-fold reduced comparative potency in vivo as an NMDA receptor antagonist. Norketamine is metabolized into dehydronorketamine and hydroxynorketamine, which are far less or negligibly active as NMDA receptor antagonists in comparison, but retain activity as potent antagonists of the α7-nicotinic acetylcholine receptor.

5β-Dihydroprogesterone is an endogenous neurosteroid and an intermediate in the biosynthesis of pregnanolone and epipregnanolone from progesterone. It is synthesized from progesterone by the enzyme 5β-reductase.
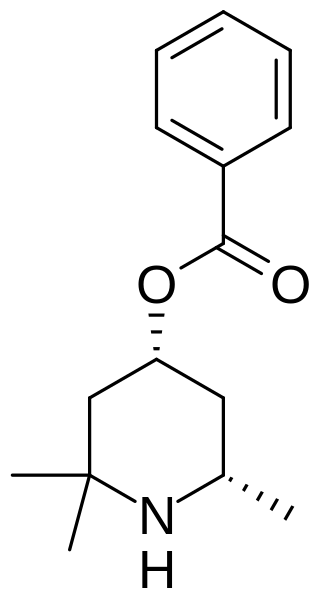
Eucaine (beta-eucaine) is a drug that was previously used as a local anesthetic. It was designed as an analog of cocaine and was one of the first synthetic chemical compounds to find general use as an anesthetic. It is a white, crystalline solid. Prior to World War I, Britain imported eucaine from Germany. During the war, a team including Jocelyn Field Thorpe and Martha Annie Whiteley developed a synthesis in Britain.
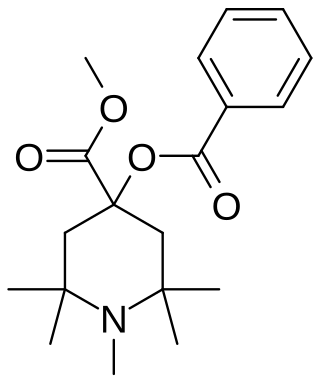
alpha-Eucaine is a drug that was previously used as a local anesthetic. It was designed as an analog of cocaine and was one of the first synthetic chemical compounds to find general use as an anesthetic.




















