
Benzodiazepines, colloquially called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, insomnia, and seizures. The first benzodiazepine, chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955, and was made available in 1960 by Hoffmann–La Roche, which followed with the development of diazepam (Valium) three years later, in 1963. By 1977, benzodiazepines were the most prescribed medications globally; the introduction of selective serotonin reuptake inhibitors (SSRIs), among other factors, decreased rates of prescription, but they remain frequently used worldwide.

Diazepam, sold under the brand name Valium among others, is a medicine of the benzodiazepine family that acts as an anxiolytic. It is used to treat a range of conditions, including anxiety, seizures, alcohol withdrawal syndrome, muscle spasms, insomnia, and restless legs syndrome. It may also be used to cause memory loss during certain medical procedures. It can be taken orally, as a suppository inserted into the rectum, intramuscularly, intravenously or used as a nasal spray. When injected intravenously, effects begin in one to five minutes and last up to an hour. When taken by mouth, effects begin after 15 to 60 minutes.

Zolpidem, sold under the brand name Ambien among others, is a medication primarily used for the short-term treatment of sleeping problems. Guidelines recommend that it be used only after cognitive behavioral therapy for insomnia and behavioral changes, such as sleep hygiene, have been tried. It decreases the time to sleep onset by about fifteen minutes and at larger doses helps people stay asleep longer. It is taken by mouth and is available in conventional tablets, sublingual tablets, or oral spray.
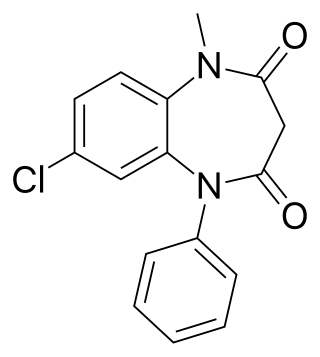
Clobazam, sold under the brand names Frisium, Onfi and others, is a benzodiazepine class medication that was patented in 1968. Clobazam was first synthesized in 1966 and first published in 1969. Clobazam was originally marketed as an anxioselective anxiolytic since 1970, and an anticonvulsant since 1984. The primary drug-development goal was to provide greater anxiolytic, anti-obsessive efficacy with fewer benzodiazepine-related side effects.

Nordazepam is a 1,4-benzodiazepine derivative. Like other benzodiazepine derivatives, it has amnesic, anticonvulsant, anxiolytic, muscle relaxant, and sedative properties. However, it is used primarily in the treatment of anxiety disorders. It is an active metabolite of diazepam, chlordiazepoxide, clorazepate, prazepam, pinazepam, and medazepam.

Clorazepate, sold under the brand name Tranxene among others, is a benzodiazepine medication. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. Clorazepate is an unusually long-lasting benzodiazepine and serves as a prodrug for the equally long-lasting desmethyldiazepam, which is rapidly produced as an active metabolite. Desmethyldiazepam is responsible for most of the therapeutic effects of clorazepate.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

Etizolam is a thienodiazepine derivative which is a benzodiazepine analog. The etizolam molecule differs from a benzodiazepine in that the benzene ring has been replaced by a thiophene ring and triazole ring has been fused, making the drug a thienotriazolodiazepine.

Pagoclone is an anxiolytic agent from the cyclopyrrolone family, related to better-known drugs such as the sleeping medication zopiclone. It was synthesized by a French team working for Rhone-Poulenc & Rorer S.A. Pagoclone belongs to the class of nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It was never commercialised.

Chlordiazepoxide, trade name Librium among others, is a sedative and hypnotic medication of the benzodiazepine class; it is used to treat anxiety, insomnia and symptoms of withdrawal from alcohol and other drugs.

Benzodiazepine withdrawal syndrome is the cluster of signs and symptoms that may emerge when a person who has been taking benzodiazepines as prescribed develops a physical dependence on them and then reduces the dose or stops taking them without a safe taper schedule.

Imidazenil is an experimental anxiolytic drug which is derived from the benzodiazepine family, and is most closely related to other imidazobenzodiazepines such as midazolam, flumazenil, and bretazenil.

QH-II-66 (QH-ii-066) is a sedative drug which is a benzodiazepine derivative. It produces some of the same effects as other benzodiazepines, but is much more selective than most other drugs of this class and so produces somewhat less sedation and ataxia than other related drugs such as diazepam and triazolam, although it still retains anticonvulsant effects.

Abecarnil (ZK-112,119) is an anxiolytic drug from the β-Carboline family. It is one of a relatively recently developed class of medicines known as the nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It is a partial agonist acting selectively at the benzodiazepine site of the GABAA receptor.
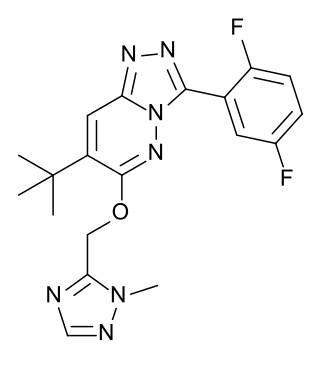
L-838,417 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. The compound was developed by Merck, Sharp and Dohme.

SL651498 is an anxiolytic and anticonvulsant drug used in scientific research, with a chemical structure most closely related to β-carboline derivatives such as abecarnil and gedocarnil. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

ELB-139 (LS-191,811) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

TPA-023 (MK-0777) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a mixed, subtype-selective ligand of the benzodiazepine site of α1, α2, α3, and α5-containing GABAA receptors, where it acts as a partial agonist at benzodiazepine sites of the α2 and α3-containing subtypes, but as a silent antagonist at α1 and α5-containing subtypes. It has primarily anxiolytic and anticonvulsant effects in animal tests, but with no sedative effects even at 50 times the effective anxiolytic dose.

TP-13 is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a subtype-selective partial agonist at GABAA receptors, binding selectively to GABAA receptor complexes bearing α2 and α3 subunits. It has modest anticonvulsant activity although less than that of diazepam, and its main effect is likely to be selective anxiolytic action, as seen with other related α2/3-preferring agonists such as L-838,417.

Benzodiazepine dependence defines a situation in which one has developed one or more of either tolerance, withdrawal symptoms, drug seeking behaviors, such as continued use despite harmful effects, and maladaptive pattern of substance use, according to the DSM-IV. In the case of benzodiazepine dependence, the continued use seems to be typically associated with the avoidance of unpleasant withdrawal reaction rather than with the pleasurable effects of the drug. Benzodiazepine dependence develops with long-term use, even at low therapeutic doses, often without the described drug seeking behavior and tolerance.




















