Salvia rosmarinus, commonly known as rosemary, is a shrub with fragrant, evergreen, needle-like leaves and white, pink, purple, or blue flowers, native to the Mediterranean region. Until 2017, it was known by the scientific name Rosmarinus officinalis, now a synonym.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

Valerian is a perennial flowering plant native to Europe and Asia. In the summer when the mature plant may have a height of 1.5 metres, it bears sweetly scented pink or white flowers that attract many fly species, especially hoverflies of the genus Eristalis. It is consumed as food by the larvae of some Lepidoptera species, including the grey pug.
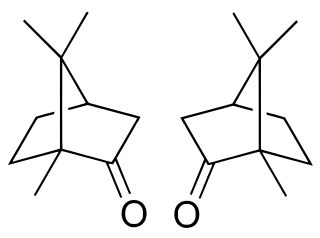
Camphor is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel, a large evergreen tree found in East Asia; and in the kapur tree, a tall timber tree from South East Asia. It also occurs in some other related trees in the laurel family, notably Ocotea usambarensis. Rosemary leaves contain 0.05 to 0.5% camphor, while camphorweed (Heterotheca) contains some 5%. A major source of camphor in Asia is camphor basil. Camphor can also be synthetically produced from oil of turpentine.

Menthol is an organic compound, more specifically a monoterpenoid, made synthetically or obtained from the oils of corn mint, peppermint, or other mints. It is a waxy, clear or white crystalline substance, which is solid at room temperature and melts slightly above.

In organic chemistry, alkenols are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The terms enol and alkenol are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves deprotonation at the α position to the carbonyl group—i.e., removal of the hydrogen atom there as a proton H+. When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate. The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as a silyl enol ether.
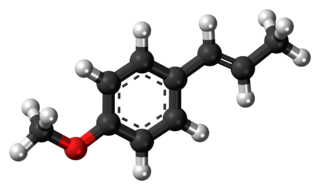
Anethole is an organic compound that is widely used as a flavoring substance. It is a derivative of phenylpropene, a type of aromatic compound that occurs widely in nature, in essential oils. It is in the class of phenylpropanoid organic compounds. It contributes a large component of the odor and flavor of anise and fennel, anise myrtle (Myrtaceae), liquorice (Fabaceae), magnolia blossoms, and star anise (Schisandraceae). Closely related to anethole is its isomer estragole, abundant in tarragon (Asteraceae) and basil (Lamiaceae), that has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid. Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This trait causes certain anise-flavored liqueurs to become opaque when diluted with water; the ouzo effect.

Eucalyptol is a monoterpenoid colorless liquid, and a bicyclic ether. It has a fresh camphor-like odor and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up about 70–90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.

The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of taxol. Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.
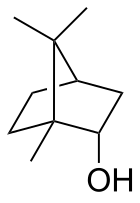
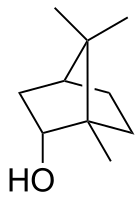
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As for other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide.
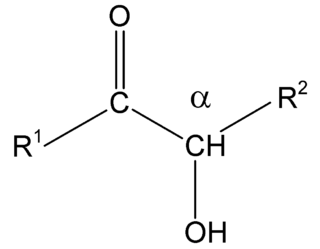
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups.
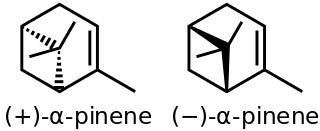
α-Pinene is an organic compound of the terpene class, one of two isomers of pinene. It is an alkene and it contains a reactive four-membered ring. It is found in the oils of many species of many coniferous trees, notably the pine. It is also found in the essential oil of rosemary and Satureja myrtifolia. Both enantiomers are known in nature; (1S,5S)- or (−)-α-pinene is more common in European pines, whereas the (1R,5R)- or (+)-α-isomer is more common in North America. The racemic mixture is present in some oils such as eucalyptus oil and orange peel oil.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.

Kaempferia galanga, commonly known as kencur, aromatic ginger, sand ginger, cutcherry, is a monocotyledonous plant in the ginger family, and one of four plants called galangal. It is found primarily in open areas in Indonesia, southern China, Taiwan, Cambodia, and India, but is also widely cultivated throughout Southeast Asia.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism refers to the existence or possibility of isomers.

1,2,3,4-Cyclohexanetetrol (also named cyclohexane-1,2,3,4-tetrol, 1,2,3,4-tetrahydroxycyclohexane, or ortho-cyclohexanetetrol) is an organic compound whose molecule can be described as a cyclohexane with four hydroxyl (OH) groups substituted for hydrogen atoms on four consecutive carbon atoms. Its formula can be written C
6H
12O
4, C
6H
8(OH)
4, or (–CH(OH)–)4(–CH
2–)2.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.