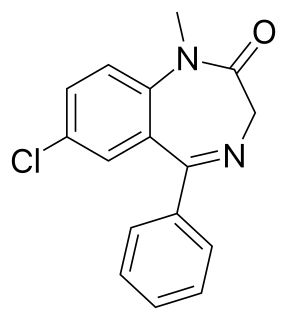
Diazepam, first marketed as Valium, is a medicine of the benzodiazepine family that acts as an anxiolytic. It is commonly used to treat a range of conditions, including anxiety, seizures, alcohol withdrawal syndrome, muscle spasms, insomnia, and restless legs syndrome. It may also be used to cause memory loss during certain medical procedures. It can be taken by mouth, inserted into the rectum, injected into muscle, injected into a vein or used as a nasal spray. When given into a vein, effects begin in one to five minutes and last up to an hour. By mouth, effects begin after 15 to 60 minutes.

Perphenazine is a typical antipsychotic drug. Chemically, it is classified as a piperazinyl phenothiazine. Originally marketed in the United States as Trilafon, it has been in clinical use for decades.

Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is an opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mouth or by injection into a vein, muscle, or under the skin. Effects generally begin within half an hour and last for up to five hours.

Amitriptyline, sold under the brand name Elavil among others, is a tricyclic antidepressant primarily used to treat cyclic vomiting syndrome (CVS), major depressive disorder and a variety of pain syndromes from neuropathic pain to fibromyalgia to migraine and tension headaches. Due to the frequency and prominence of side effects, amitriptyline is generally considered a second-line therapy for these indications.
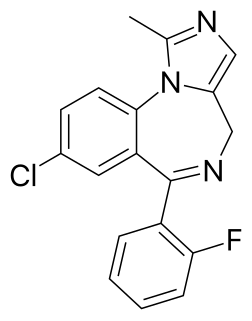
Midazolam, sold under the brand name Versed among others, is a benzodiazepine medication used for anesthesia and procedural sedation, and to treat severe agitation. It works by inducing sleepiness, decreasing anxiety, and causing a loss of ability to create new memories. The drug does not cause an individual to become unconscious, merely to be sedated. It is also useful for the treatment of prolonged seizures. Midazolam can be given by mouth, intravenously, by injection into a muscle, by spraying into the nose, or through the cheek. When given intravenously, it typically begins working within five minutes; when injected into a muscle, it can take fifteen minutes to begin working. Effects last between one and six hours.

Lithium orotate (C5H3LiN2O4) is a salt of orotic acid and lithium. It is available as the monohydrate, LiC5H3N2O4·H2O. In this compound, lithium is non-covalently bound to an orotate ion, rather than to a carbonate or other ion, and like other salts, dissociates in solution to produce free lithium ions. It is marketed as a dietary supplement, though it has been researched minimally between 1973–1986 to treat certain medical conditions, such as alcoholism and Alzheimer's disease.
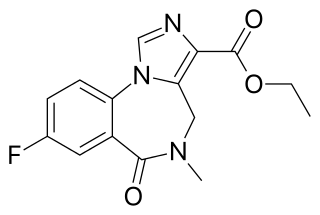
Flumazenil is a selective GABAA receptor antagonist administered via injection, otic insertion, or intranasally. Therapeutically, it acts as both an antagonist and antidote to benzodiazepines, through competitive inhibition.

Zopiclone, sold under the brand name Imovane among others, is a nonbenzodiazepine used to treat difficulty sleeping. Zopiclone is molecularly distinct from benzodiazepine drugs and is classed as a cyclopyrrolone. However, zopiclone increases the normal transmission of the neurotransmitter gamma-aminobutyric acid (GABA) in the central nervous system, via modulating GABAA receptors similarly to the way benzodiazepine drugs do.

Bromazepam, sold under many brand names, is a benzodiazepine. It is mainly an anti-anxiety agent with similar side effects to diazepam (Valium). In addition to being used to treat anxiety or panic states, bromazepam may be used as a premedicant prior to minor surgery. Bromazepam typically comes in doses of 3 mg and 6 mg tablets.
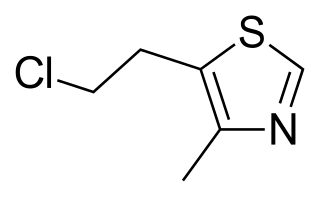
Clomethiazole is a sedative and hypnotic originally developed by Hoffmann-La Roche in the 1930s. The drug is used in treating and preventing symptoms of acute alcohol withdrawal.

Cinnarizine is an antihistamine and calcium channel blocker of the diphenylmethylpiperazine group. It is prescribed for nausea and vomiting due to motion sickness or other sources such as chemotherapy, vertigo, or Ménière's disease.

Sulpiride, sold under the brand name Dogmatil among others, is an atypical antipsychotic medication of the benzamide class which is used mainly in the treatment of psychosis associated with schizophrenia and major depressive disorder, and sometimes used in low dosage to treat anxiety and mild depression. Sulpiride is commonly used in Asia, Central America, Europe, South Africa and South America. Levosulpiride is its purified levo-isomer and is sold in India for similar purpose. It is not approved in the United States, Canada, or Australia. The drug is chemically and clinically similar to amisulpride.

Flupentixol (INN), also known as flupenthixol, marketed under brand names such as Depixol and Fluanxol is a typical antipsychotic drug of the thioxanthene class. It was introduced in 1965 by Lundbeck. In addition to single drug preparations, it is also available as flupentixol/melitracen—a combination product containing both melitracen and flupentixol. Flupentixol is not approved for use in the United States. It is, however, approved for use in the UK, Australia, Canada, Russian Federation, South Africa, New Zealand, Philippines and various other countries.

Acecainide is an antiarrhythmic drug. Chemically, it is the N-acetylated metabolite of procainamide. It is a Class III antiarrhythmic agent, whereas procainamide is a Class Ia antiarrhythmic drug. It is only partially as active as procainamide; when checking levels, both must be included in the final calculation.
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.

Delorazepam, also known as chlordesmethyldiazepam and nordiclazepam, is a drug which is a benzodiazepine and a derivative of desmethyldiazepam. It is marketed in Italy, where it is available under the trade name EN and Dadumir. Delorazepam (chlordesmethyldiazepam) is also an active metabolite of the benzodiazepine drugs diclazepam and cloxazolam. Adverse effects may include hangover type effects, drowsiness, behavioural impairments and short-term memory impairments. Similar to other benzodiazepines delorazepam has anxiolytic, skeletal muscle relaxant, hypnotic and anticonvulsant properties.

Deramciclane (EGIS-3886) is a non-benzodiazepine-type anxiolytic drug to treat various types of anxiety disorders. Deramciclane is a unique alternative to current anxiolytics on the market because it has a novel chemical structure and target. It acts as an antagonist at the 5-HT2A receptor, as an inverse agonist at the 5-HT2C receptor, and as a GABA reuptake inhibitor. The two serotonin receptors are G protein-coupled receptors and are two of the main excitatory serotonin receptor types. Their excitation has been implicated in anxiety and mood. Deramciclane does not affect CYP3A4 activity in metabolizing other drugs, but it is a weak inhibitor of CYP2D6. Some studies also show the drug to have moderate affinity to dopamine D2 receptors and low affinity to dopamine receptor D1. Researchers are looking for alternatives to benzodiazepines for anxiolytic use because benzodiazepine drugs have sedative and muscle relaxant side effects.

Lirequinil (Ro41-3696) is a nonbenzodiazepine hypnotic drug which binds to benzodiazepine sites on the GABAA receptor. In human clinical trials, lirequinil was found to have similar efficacy to zolpidem, with less side effects such as clumsiness and memory impairment. However it was also much slower acting than zolpidem, with peak plasma concentrations not reached until 2.5 hours after oral administration, and its O-desethyl metabolite Ro41-3290 is also active with a half-life of 8 hours. This meant that while effective as a hypnotic, lirequinil failed to prove superior to zolpidem due to producing more next-day sedation, and it has not been adopted for clinical use. It was developed by a team at Hoffmann-La Roche in the 1990s.

Dapoxetine, marketed as Priligy, among others, is a medication used for the treatment of premature ejaculation (PE) in men 18–64 years old. Dapoxetine works by inhibiting the serotonin transporter, increasing serotonin's action at the postsynaptic cleft, and as a consequence promoting ejaculatory delay. As a member of the selective serotonin reuptake inhibitor (SSRI) family, dapoxetine was initially created as an antidepressant. However, unlike other SSRIs, dapoxetine is absorbed and eliminated rapidly in the body. Its fast-acting property makes it suitable for the treatment of PE, but not as an antidepressant.



















