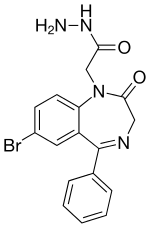
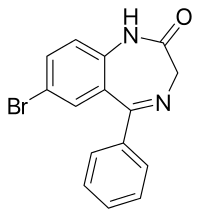
An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

Buspirone, sold under the brand name Buspar, among others, is an anxiolytic, a medication primarily used to treat anxiety disorders, particularly generalized anxiety disorder. It is a serotonin 5-HT1A receptor agonist, increasing action at serotonin receptors in the brain. It is taken orally, and takes two to six weeks to be fully effective.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

Medazepam is a drug that is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative, and skeletal muscle relaxant properties. It is known by the following brand names: Azepamid, Nobrium, Tranquirax, Rudotel, Raporan, Ansilan and Mezapam. Medazepam is a long-acting benzodiazepine drug. The half-life of medazepam is 36–200 hours.

Prazepam is a benzodiazepine derivative drug developed by Warner-Lambert in the 1960s. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Prazepam is a prodrug for desmethyldiazepam which is responsible for the therapeutic effects of prazepam.

Ethyl loflazepate is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses. Its elimination half-life is 51–103 hours. Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound. Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate. The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate. Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment. The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children. Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity. High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.
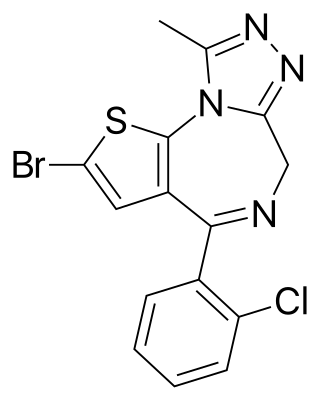
Brotizolam is a sedative-hypnotic thienotriazolodiazepine drug which is a benzodiazepine analog. It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties, and is considered to be similar in effect to other short-acting hypnotic benzodiazepines such as triazolam or midazolam. It is used in the short-term treatment of severe insomnia. Brotizolam is a highly potent and short-acting hypnotic, with a typical dose ranging from 0.125 to 0.25 milligrams, which is rapidly eliminated with an average half-life of 4.4 hours.

Picamilon is a drug formed by a synthetic combination of niacin and γ-aminobutyric acid (GABA). It was developed in the Soviet Union in 1969 and further studied in both Russia and Japan as a prodrug of GABA.

Acecainide is an antiarrhythmic drug. Chemically, it is the N-acetylated metabolite of procainamide. It is a Class III antiarrhythmic agent, whereas procainamide is a Class Ia antiarrhythmic drug. It is only partially as active as procainamide; when checking levels, both must be included in the final calculation.
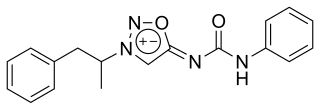
Mesocarb is a drug that is currently being developed for Parkinson's disease.

Delorazepam, also known as chlordesmethyldiazepam and nordiclazepam, is a drug which is a benzodiazepine and a derivative of desmethyldiazepam. It is marketed in Italy, where it is available under the trade name EN and Dadumir. Delorazepam (chlordesmethyldiazepam) is also an active metabolite of the benzodiazepine drugs diclazepam and cloxazolam. Adverse effects may include hangover type effects, drowsiness, behavioural impairments and short-term memory impairments. Similar to other benzodiazepines delorazepam has anxiolytic, skeletal muscle relaxant, hypnotic and anticonvulsant properties.

Fosazepam is a drug which is a benzodiazepine derivative; it is a water soluble derivative of diazepam. It has sedative and anxiolytic effects, and is a derivative of diazepam which has been substituted with a dimethylphosphoryl group to improve solubility in water.
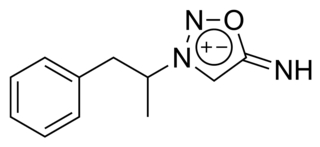
Feprosidnine (Sydnophen) is a stimulant drug which was developed in the USSR in the 1970s. It is structurally related to another Russian drug mesocarb but unlike mesocarb, was withdrawn earlier from production. In comparison with mesocarb it has own antidepressant activity, which makes it useful in treating depressions. Indications of feprosidnine included apathic, asthenic depressions, fatigue, apathic syndrome, narcolepsy and other similar conditions. Therapeutic range of doses: 10-50mg a day. Sydnophen has multiple mechanisms of action, the relative importance of which has not been clearly established. Effects on the body include reversible monoamine oxidase inhibition, cholinergic, adrenergic, opioid and nitric oxide donating actions, all of which may contribute to its pharmacological effects to some extent.

Bromantane, sold under the brand name Ladasten, is an atypical psychostimulant and anxiolytic drug of the adamantane family related to amantadine and memantine which is used in Russia in the treatment of neurasthenia. Although the effects of the bromantane have been determined to be dependent on the dopaminergic and possibly serotonergic neurotransmitter systems, its exact mechanism of action is unknown, and it is distinct in its properties relative to typical psychostimulants such as amphetamine. Because of its unique aspects, bromantane has sometimes been described instead as an adaptogen and actoprotector.

Fabomotizole is an anxiolytic drug launched in Russia in the early 2000s. It produces anxiolytic and neuroprotective effects without any sedative or muscle relaxant actions. Its mechanism of action remains poorly defined however, with GABAergic, NGF- and BDNF-release-promoting, MT1 receptor agonism, MT3 receptor antagonism, and sigma agonism suggested as potential mechanisms. Fabomotizole was shown to inhibit MAO-A reversibly and there might be also some involvement with serotonin receptors. Clinical trials have shown fabomotizole to be well tolerated and reasonably effective for the treatment of anxiety.

Selank is a nootropic, anxiolytic peptide based drug developed by the Institute of Molecular Genetics of the Russian Academy of Sciences. Selank is a heptapeptide with the sequence Thr-Lys-Pro-Arg-Pro-Gly-Pro (TKPRPGP). It is a synthetic analogue of human tuftsin.

Emoxypine (2-ethyl-6-methyl-3-hydroxypyridine), also known as Mexidol or Mexifin when used as the succinate salt, is an antioxidant actoprotector manufactured by Pharmasoft Pharmaceuticals. Its chemical structure resembles that of pyridoxine (a type of vitamin B6).

Nooglutyl is a nootropic agent that was studied at the Research Institute of Pharmacology, Russian Academy of Medical Sciences as a potential treatment for amnesia.

Cinazepam is an atypical benzodiazepine derivative. It produces pronounced hypnotic, sedative, and anxiolytic effects with minimal myorelaxant side effects. In addition, unlike many other benzodiazepine and nonbenzodiazepine hypnotics such as diazepam, flunitrazepam, and zopiclone, cinazepam does not violate sleep architecture, and the continuity of slow-wave sleep and REM sleep are proportionally increased. As such, cinazepam produces a sleep state close to physiological, and for that reason, may be advantageous compared to other, related drugs in the treatment of insomnia and other sleep disorders.

Tolibut, also known as 3-(p-tolyl)-4-aminobutyric acid (or β-(4-methylphenyl)-GABA), is drug that was developed in Russia. It is an analogue of γ-aminobutyric acid (GABA) (that is, a GABA analogue) and is the 4-methyl analogue of phenibut, and is also an analogue of baclofen where the 4-chloro substitution has been replaced with a 4-methyl substitution. Tolibut has been described as possessing analgesic, tranquilizing, and neuroprotective properties. It is not fully clear as to whether the drug was ever approved or used medically in Russia.