
Flunitrazepam, also known as Rohypnol among other names, is a benzodiazepine used to treat severe insomnia and assist with anesthesia. As with other hypnotics, flunitrazepam has been advised to be prescribed only for short-term use or by those with chronic insomnia on an occasional basis.
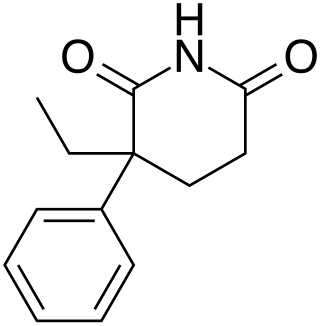
Glutethimide is a hypnotic sedative that was introduced by Ciba in 1954 as a safe alternative to barbiturates to treat insomnia. Before long, however, it had become clear that glutethimide was just as likely to cause addiction and caused similar withdrawal symptoms. Doriden was the brand-name version. Current production levels in the United States point to its use only in small-scale research. Manufacturing of the drug was discontinued in the US in 1993 and discontinued in several eastern European countries in 2006.

A sedative or tranquilliser is a substance that induces sedation by reducing irritability or excitement. They are CNS depressants and interact with brain activity causing its deceleration. Various kinds of sedatives can be distinguished, but the majority of them affect the neurotransmitter gamma-aminobutyric acid (GABA). In spite of the fact that each sedative acts in its own way, most produce relaxing effects by increasing GABA activity.

Zopiclone, sold under the brand name Imovane among others, is a nonbenzodiazepine used to treat difficulty sleeping. Zopiclone is molecularly distinct from benzodiazepine drugs and is classed as a cyclopyrrolone. However, zopiclone increases the normal transmission of the neurotransmitter gamma-aminobutyric acid (GABA) in the central nervous system, via modulating GABAA receptors similarly to the way benzodiazepine drugs do.

Nitrazepam, sold under the brand name Mogadon among others, is a hypnotic drug of the benzodiazepine class used for short-term relief from severe, disabling anxiety and insomnia. It also has sedative (calming) properties, as well as amnestic, anticonvulsant, and skeletal muscle relaxant effects.

Zaleplon, sold under the brand name Sonata among others, is a sedative and hypnotic which is used to treat insomnia. It is a nonbenzodiazepine or Z-drug of the pyrazolopyrimidine class. It was developed by King Pharmaceuticals and approved for medical use in the United States in 1999.
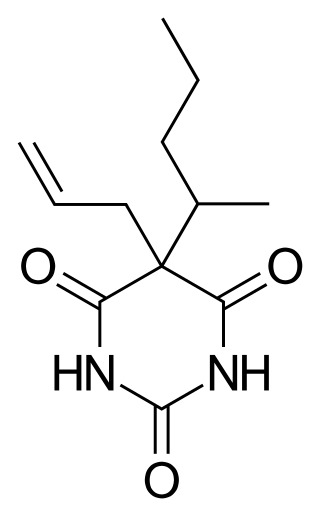
Secobarbital is a short-acting barbiturate derivative drug that was patented in 1934 in the United States. It possesses anaesthetic, anticonvulsant, anxiolytic, sedative, and hypnotic properties. In the United Kingdom, it was known as quinalbarbitone. It is the most frequently used drug in physician-assisted suicide within the United States. Secobarbital is considered to be an obsolete sedative-hypnotic, and as a result, it has largely been replaced by the benzodiazepine family. Seconal was widely abused, known on the street as "red devils" or "reds".

Quazepam, sold under brand name Doral among others, is a relatively long-acting benzodiazepine derivative drug developed by the Schering Corporation in the 1970s. Quazepam is used for the treatment of insomnia including sleep induction and sleep maintenance. Quazepam induces impairment of motor function and has relatively selective hypnotic and anticonvulsant properties with considerably less overdose potential than other benzodiazepines. Quazepam is an effective hypnotic which induces and maintains sleep without disruption of the sleep architecture.
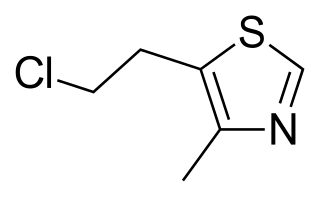
Clomethiazole is a sedative and hypnotic originally developed by Hoffmann-La Roche in the 1930s. The drug is used in treating and preventing symptoms of acute alcohol withdrawal.

Lormetazepam, sold under the brand name Noctamid among others, is a drug which is a short to intermediate acting 3-hydroxy benzodiazepine derivative and temazepam analogue. It possesses hypnotic, anxiolytic, anticonvulsant, sedative, and skeletal muscle relaxant properties.

Methyprylon, or Noludar, is a sedative of the piperidinedione derivative family first developed by Hoffmann-La Roche. This medicine was used for treating insomnia, but is now rarely used as it has been replaced by newer drugs with fewer side effects, such as benzodiazepines.
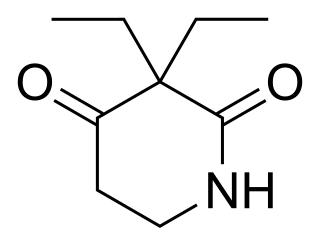
Piperidione is a sedative drug, structurally related to methyprylon and pyrithyldione. It used to be marketed by Roche as a cough medicine available in liquid form. In the US, it was approved by the FDA on grounds of safety alone in 1947. After Roche failed to submit evidence of efficacy to the Drug Efficacy Study Implementation program in 1972, it was withdrawn from the US market.

A GABA receptor agonist is a drug that is an agonist for one or more of the GABA receptors, producing typically sedative effects, and may also cause other effects such as anxiolytic, anticonvulsant, and muscle relaxant effects. There are three receptors of the gamma-aminobutyric acid. The two receptors GABA-α and GABA-ρ are ion channels that are permeable to chloride ions which reduces neuronal excitability. The GABA-β receptor belongs to the class of G-Protein coupled receptors that inhibit adenylyl cyclase, therefore leading to decreased cyclic adenosine monophosphate (cAMP). GABA-α and GABA-ρ receptors produce sedative and hypnotic effects and have anti-convulsion properties. GABA-β receptors also produce sedative effects. Furthermore, they lead to changes in gene transcription.
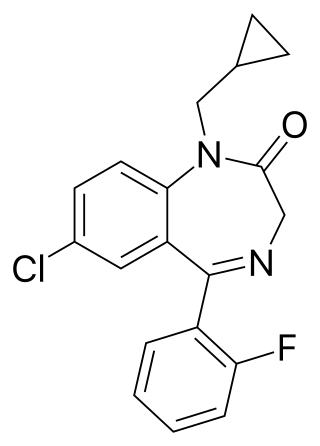
Flutoprazepam (Restas) is a drug which is a benzodiazepine. It was patented in Japan by Sumitomo in 1972 and its medical use remains mostly confined to that country. Its muscle relaxant properties are approximately equivalent to those of diazepam - however, it has more powerful sedative, hypnotic, anxiolytic and anticonvulsant effects and is around four times more potent by weight compared to diazepam. It is longer acting than diazepam due to its long-acting active metabolites, which contribute significantly to its effects. Its principal active metabolite is n-desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam.

CL-218,872 is a sedative and hypnotic drug used in scientific research. It has similar effects to sedative-hypnotic benzodiazepine drugs such as triazolam, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic.

Umespirone (KC-9172) is a drug of the azapirone class which possesses anxiolytic and antipsychotic properties. It behaves as a 5-HT1A receptor partial agonist (Ki = 15 nM), D2 receptor partial agonist (Ki = 23 nM), and α1-adrenoceptor receptor antagonist (Ki = 14 nM), and also has weak affinity for the sigma receptor (Ki = 558 nM). Unlike many other anxiolytics and antipsychotics, umespirone produces minimal sedation, cognitive/memory impairment, catalepsy, and extrapyramidal symptoms.
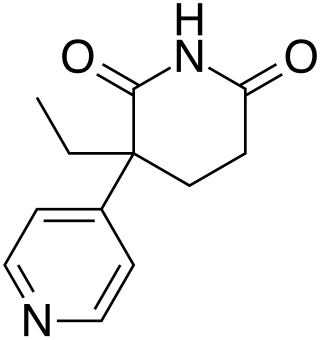
Rogletimide, also known as pyridoglutethimide, is a medication which was never marketed. It is related in chemical structure to the sedative/hypnotic drug glutethimide, but instead has pharmacological activity as a selective aromatase inhibitor similar to the related drug aminoglutethimide and has no significant sedative-hypnotic effect. This makes it potentially useful in the treatment of breast cancer, and with fewer side effects than aminoglutethimide, but its lower potency caused it to be unsuccessful in clinical trials.

Lirequinil (Ro41-3696) is a nonbenzodiazepine hypnotic drug which binds to benzodiazepine sites on the GABAA receptor. In human clinical trials, lirequinil was found to have similar efficacy to zolpidem, with less side effects such as clumsiness and memory impairment. However it was also much slower acting than zolpidem, with peak plasma concentrations not reached until 2.5 hours after oral administration, and its O-desethyl metabolite Ro41-3290 is also active with a half-life of 8 hours. This meant that while effective as a hypnotic, lirequinil failed to prove superior to zolpidem due to producing more next-day sedation, and it has not been adopted for clinical use. It was developed by a team at Hoffmann-La Roche in the 1990s.

JM-1232 is a sedative and hypnotic drug being researched as a potential anesthetic. It has similar effects to sedative-hypnotic benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic. It was developed by a team at Maruishi Pharmaceutica.

Fluperlapine, also known as fluoroperlapine, is a morphanthridine (11H-dibenzo[b,e]azepine) atypical antipsychotic with additional antidepressant and sedative effects. It was first synthesized in 1979, and then subsequently studied in animals and humans in 1984 and beyond, but despite demonstrating efficacy in the treatment of a variety of medical conditions including schizophrenia, psychosis associated with Parkinson's disease, depressive symptoms, and dystonia, it was never marketed. This was perhaps due to its capacity for producing potentially life-threatening agranulocytosis, similarly to clozapine, which it closely resembles both structurally and pharmacologically.





















