
Carbamazepine (CBZ), sold under the trade name Tegretol among others, is an anticonvulsant medication used primarily in the treatment of epilepsy and neuropathic pain. It is used in schizophrenia along with other medications and as a second-line agent in bipolar disorder. Carbamazepine appears to work as well as phenytoin and valproate for focal and generalized seizures. It is not effective for absence or myoclonic seizures.
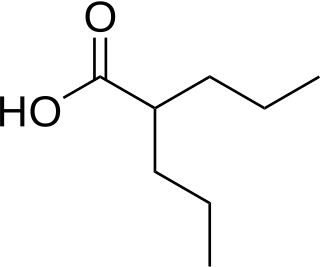
Valproate (VPA) and its valproic acid, sodium valproate, and valproate semisodium forms are medications primarily used to treat epilepsy and bipolar disorder and prevent migraine headaches. They are useful for the prevention of seizures in those with absence seizures, partial seizures, and generalized seizures. They can be given intravenously or by mouth, and the tablet forms exist in both long- and short-acting formulations.
Anticonvulsants are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain.

Lamotrigine, sold as the brand name Lamictal among others, is an anticonvulsant medication used to treat epilepsy and to delay or prevent the recurrence of depressive episodes in bipolar disorder. For epilepsy, this includes focal seizures, tonic-clonic seizures, and seizures in Lennox-Gastaut syndrome. In bipolar disorder, lamotrigine has not been shown to reliably treat acute depression; but for patients with bipolar disorder who are not currently symptomatic, it appears to be effective in reducing the risk of future episodes of depression.
Absence seizures are one of several kinds of generalized seizures. These seizures are sometimes referred to as petit mal seizures. Absence seizures are characterized by a brief loss and return of consciousness, generally not followed by a period of lethargy.

Oxcarbazepine, sold under the brand name Trileptal among others, is a medication used to treat epilepsy and bipolar disorder. For epilepsy it is used for both focal seizures and generalized seizures. It has been used both alone and as add-on therapy in people with bipolar who have had no success with other treatments. It is taken by mouth.
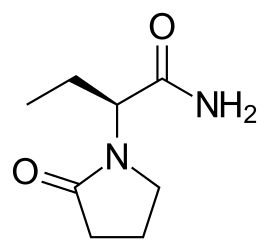
Levetiracetam, sold under the brand name Keppra among others, is a medication used to treat epilepsy. It is used for partial-onset, myoclonic, or tonic–clonic seizures and is taken either by mouth as an immediate or extended release formulation or by injection into a vein.

Valpromide is a carboxamide derivative of valproic acid used in the treatment of epilepsy and some affective disorders. It is rapidly metabolised (80%) to valproic acid but has anticonvulsant properties itself. It may produce more stable plasma levels than valproic acid or sodium valproate and may be more effective at preventing febrile seizures. However, it is over one hundred times more potent as an inhibitor of liver microsomal epoxide hydrolase. This makes it incompatible with carbamazepine and can affect the ability of the body to remove other toxins. Valpromide is no safer during pregnancy than valproic acid.

Cannabidivarin (CBDV) is a non-psychoactive cannabinoid found in Cannabis. It is a homolog of cannabidiol (CBD), with the side-chain shortened by two methylene bridges (CH2 units). Plants with relatively high levels of CBDV have been reported in feral populations of C. indica ( = C. sativa ssp. indica var. kafiristanica) from northwest India, and in hashish from Nepal. CBDV has anticonvulsant effects. It was identified for the first time in 1969 by Vollner et al.

Primidone, sold under various brand names, is a barbiturate medication that is used to treat partial and generalized seizures, as well as essential tremors. It is taken by mouth.
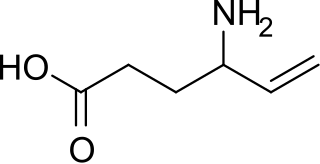
Vigabatrin, brand name Sabril, is a medication used to treat epilepsy. It became available as a generic medication in 2019.
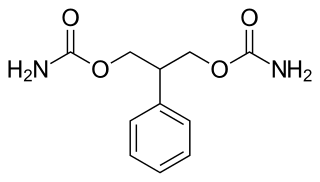
Felbamate is an anticonvulsant used in the treatment of epilepsy. It is used to treat partial seizures in adults and partial and generalized seizures associated with Lennox–Gastaut syndrome in children. However, an increased risk of potentially fatal aplastic anemia and/or liver failure limit the drug's usage to severe refractory epilepsy.

Seletracetam is a pyrrolidone-derived drug of the racetam family that is structurally related to levetiracetam. It was under development by UCB Pharmaceuticals as a more potent and effective anticonvulsant drug to replace levetiracetam but its development has been halted.

Brivaracetam, sold under the brand name Briviact among others, a chemical analog of levetiracetam, is a racetam derivative with anticonvulsant (antiepileptic) properties. It is marketed by the pharmaceutical company UCB. In India it is co-promoted and distributed by Dr. Reddy's Laboratories.

Lacosamide, sold under the brand name Vimpat among others, is a medication used in the adjunctive treatment of partial-onset seizures and diabetic neuropathic pain. It is used by mouth or intravenously.

Remacemide is a drug which acts as a low-affinity NMDA antagonist with sodium channel blocking properties. It has been studied for the treatment of acute ischemic stroke, epilepsy, Huntington's disease, and Parkinson's disease.

Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production has been discontinued in June 2017.
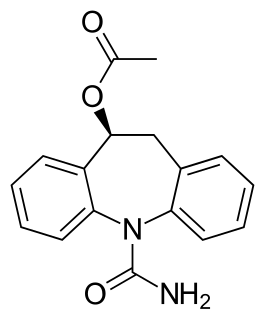
Eslicarbazepine acetate (ESL), sold under the brand names Aptiom and Zebinix among others, is an anticonvulsant medication approved for use in Europe and the United States as monotherapy or as additional therapy for partial-onset seizures epilepsy.

Talampanel is a drug which has been investigated for the treatment of epilepsy, malignant gliomas, and amyotrophic lateral sclerosis (ALS).

Nordoxepin, also known as N-desmethyldoxepin, is the major active metabolite of the tricyclic antidepressant (TCA) doxepin (Sinequan). It has been found to play a significant role in the antidepressant effects of doxepin.




















