Colloquially known as "downers," depressants, or central depressants, are drugs that lower neurotransmission levels, or depress or reduce arousal or stimulation in various areas of the brain. Depressants do not change the mood or mental state of others. Stimulants, or "uppers," increase mental or physical function, hence the opposite drug class from depressants are stimulants, not antidepressants.

Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat partial seizures and neuropathic pain. It is commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. It is moderately effective: about 30–40% of those given gabapentin for diabetic neuropathy or postherpetic neuralgia have a meaningful benefit.
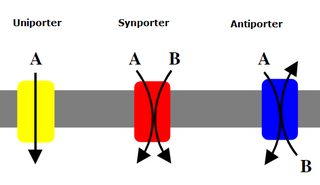
Uniporters, also known as solute carriers or facilitated transporters, are a type of membrane transport protein that passively transports solutes across a cell membrane. It uses facilitated diffusion for the movement of solutes down their concentration gradient from an area of high concentration to an area of low concentration. Unlike active transport, it does not require energy in the form of ATP to function. Uniporters are specialized to carry one specific ion or molecule and can be categorized as either channels or carriers. Facilitated diffusion may occur through three mechanisms: uniport, symport, or antiport. The difference between each mechanism depends on the direction of transport, in which uniport is the only transport not coupled to the transport of another solute.

β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.

Pregabalin, sold under the brand name Lyrica among others, is an anticonvulsant, analgesic and anxiolytic medication used to treat epilepsy, neuropathic pain, fibromyalgia, restless leg syndrome, opioid withdrawal and generalized anxiety disorder (GAD). Pregabalin also has antiallodynic properties. Its use in epilepsy is as an add-on therapy for partial seizures. It is a gabapentinoid medication and acts by inhibiting certain calcium channels. When used before surgery, it reduces pain but results in greater sedation and visual disturbances. It is taken by mouth.

Hartnup disease is an autosomal recessive metabolic disorder affecting the absorption of nonpolar amino acids. Niacin is a precursor to nicotinamide, a necessary component of NAD+.

A dopamine agonist(DA) is a compound that activates dopamine receptors. There are two families of dopamine receptors, D1-like and D2-like. They are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used in the treatment of Parkinson's disease, and to a lesser extent, in hyperprolactinemia and restless legs syndrome. They are also used off-label in the treatment of clinical depression. The use of dopamine agonists is associated with impulse control disorders and dopamine agonist withdrawal syndrome (DAWS).

Phenibut, sold under the brand names Anvifen, Fenibut, and Noofen among others, is a central nervous system depressant with anxiolytic effects, and is used to treat anxiety, insomnia, and for a variety of other indications. It is usually taken by mouth as a tablet, but may be given intravenously.

Endomorphins are considered to be natural opioid neurotransmitters central to pain relief. The two known endomorphins, endomorphin-1 and endomorphin-2, are tetrapeptides, consisting of Tyr-Pro-Trp-Phe and Tyr-Pro-Phe-Phe amino acid sequences respectively. These sequences fold into tertiary structures with high specificity and affinity for the μ-opioid receptor, binding it exclusively and strongly. Bound μ-opioid receptors typically induce inhibitory effects on neuronal activity. Endomorphin-like immunoreactivity exists within the central and peripheral nervous systems, where endomorphin-1 appears to be concentrated in the brain and upper brainstem, and endomorphin-2 in the spinal cord and lower brainstem. Because endomorphins activate the μ-opioid receptor, which is the target receptor of morphine and its derivatives, endomorphins possess significant potential as analgesics with reduced side effects and risk of addiction.

Quisqualic acid is an agonist of the AMPA, kainate, and group I metabotropic glutamate receptors. It is one of the most potent AMPA receptor agonists known. It causes excitotoxicity and is used in neuroscience to selectively destroy neurons in the brain or spinal cord. Quisqualic acid occurs naturally in the seeds of Quisqualis species.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

PD-217,014 is a drug developed by Pfizer and related to gabapentin, which similarly binds to the α2δ calcium channels. It was developed as a potentially more potent successor to gabapentin and pregabalin, along with several other analogues such as atagabalin and 4-methylpregabalin, but while PD-217,014 produces visceral analgesic effects in animal studies with higher potency and efficacy than gabapentin, it was not developed further for clinical use because of its comparatively more complex synthesis, compared to other related analogues.
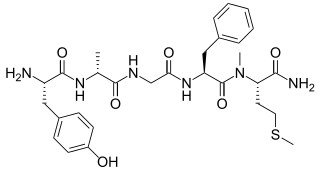
Metkefamide (INN; LY-127,623), or metkephamid acetate (USAN), but most frequently referred to simply as metkephamid, is a synthetic opioid pentapeptide and derivative of [Met]enkephalin with the amino acid sequence Tyr-D-Ala-Gly-Phe-(N-Me)-Met-NH2. It behaves as a potent agonist of the δ- and μ-opioid receptors with roughly equipotent affinity, and also has similarly high affinity as well as subtype-selectivity for the κ3-opioid receptor.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

Gabapentinoids, also known as α2δ ligands, are a class of drugs that are derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) which block α2δ subunit-containing voltage-dependent calcium channels (VDCCs). This site has been referred to as the gabapentin receptor, as it is the target of the drugs gabapentin and pregabalin.

Endomorphin-1 (EM-1) (amino acid sequence Tyr-Pro-Trp-Phe-NH2) is an endogenous opioid peptide and one of the two endomorphins. It is a high affinity, highly selective agonist of the μ-opioid receptor, and along with endomorphin-2 (EM-2), has been proposed to be the actual endogenous ligand of the μ-receptor. EM-1 produces analgesia in animals and is equipotent with morphine in this regard. The gene encoding for EM-1 has not yet been identified, and it has been suggested that endomorphins could be synthesized by an enzymatic, non-ribosomal mechanism.
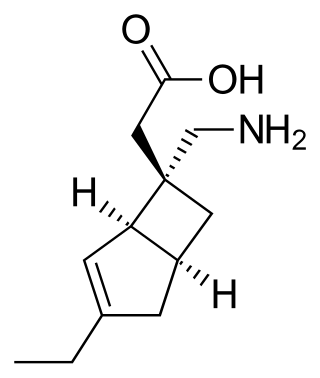
Mirogabalin is a gabapentinoid medication developed by Daiichi Sankyo. Gabapentin and pregabalin are also members of this class. As a gabapentinoid, mirogabalin binds to the α2δ subunit of voltage-gated calcium channel, but with significantly higher potency than pregabalin. It has shown promising results in Phase II clinical trials for the treatment of diabetic peripheral neuropathic pain.
Members of the Organo Anion Transporter (OAT) Family are membrane transport proteins or 'transporters' that mediate the transport of mainly organic anions across the cell membrane. Therefore, OATPs are present in the lipid bilayer of the cell membrane, acting as the cell's gatekeepers. OATPs belong to the Solute Carrier Family (SLC) and the major facilitator superfamily.
Peripherally acting μ-opioid receptor antagonists (PAMORAs) are a class of chemical compounds that are used to reverse adverse effects caused by opioids interacting with receptors outside the central nervous system (CNS), mainly those located in the gastrointestinal tract. PAMORAs are designed to specifically inhibit certain opioid receptors in the gastrointestinal tract and with limited ability to cross the blood–brain barrier. Therefore, PAMORAs do not affect the analgesic effects of opioids within the central nervous system.
Proton-coupled amino acid transporters belong to the SLC26A5 family; they are protein receptors whose main function is the transmembrane movement of amino acids and their derivatives. This family of receptors is most commonly found within the luminal surface of the small intestine as well as in some lysosomes. The solute carrier family (SLC) of genes includes roughly 400 membrane proteins that are characterized by 66 families in total. The SLC36 family of genes maps to chromosome 11. The diversity of these receptors is vast, with the ability to transport both charged and uncharged amino acids along with their derivatives. In research and practice, SLC36A1/2 are both targets for drug-based delivery systems for a wide range of disorders.














