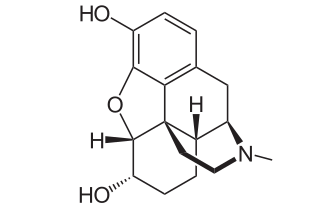
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Dipipanone, sold under the brand names of Pipadone and Diconal is a strong opioid analgesic drug, used for acute pain by mouth (PO) for adults. It is often used in instances where morphine is indicated but cannot be used due to the patient being allergic to morphine. In analgesic potency 25 mg dipipanone is approximately equivalent to 10 mg morphine.

Dextromoramide is a powerful opioid analgesic approximately three times more potent than morphine but shorter acting. It is subject to drug prohibition regimes, both internationally through UN treaties and by the criminal law of individual nations, and is usually prescribed only in the Netherlands.

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.

Properidine is an opioid, an analgesic, and the isopropyl analog of pethidine. Properidine is under international control and is listed in the United States under the Controlled Substances Act (1970) as a Schedule I substance. It is a narcotic, with an Administrative Controlled Substances Code Number (ACSCN) of 9644 and a 2 gramme annual aggregate manufacturing quota as of 2014. The salt in use is hydrochloride, with a free base conversion ratio of 0.88.

Metopon is an opioid analogue that is a methylated derivative of hydromorphone which was invented in 1929 as an analgesic.

Diethylthiambutene is an opioid analgesic drug developed in the 1950s which was mainly used as an anesthetic in veterinary medicine and continues, along with the other two thiambutenes dimethylthiambutene and ethylmethylthiambutene to be used for this purpose, particularly in Japan. It is now under international control under Schedule I of the UN Single Convention On Narcotic Drugs 1961, presumably due to high abuse potential, although little more information is available. It is listed under Schedule I of the US Controlled Substances Act as a Narcotic and has an ACSCN of 9616 with zero annual manufacturing quota as of 2013.

Dimethylthiambutene (N,N-Dimethyl-1-methyl-3,3-di-2-thienylallylamine, DMTB, trade names Ohton, Aminobutene, Dimethibutin, Kobaton, Takaton, Dimethibutin) is an opioid analgesic drug, most often used in veterinary medicine in Japan and to a lesser extent in other countries in the region and around the world. It is the most prominent and widely used of the thiambutenes, a series of open-chain opioids structurally related to methadone which are also called the thienyl derivative opioids which also includes diethylthiambutene and ethylmethylthiambutene, as well as the non-opioid cough suppressant tipepidine.
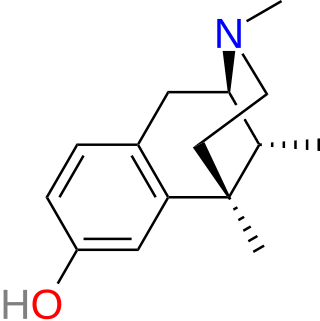
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Diampromide is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and phenampromide. It was invented in the 1960s by American Cyanamid, and can be described as a ring-opened analogue of fentanyl.

Pethidine intermediate A is a 4-phenylpiperidine derivative that is a precursor to the opioid analgesic drug pethidine (meperidine). It is not known to have any analgesic activity in its own right, however other derivatives of pethidine with a 4-cyano group in place of the carboxylate ethyl ester have been found to be active, so pethidine intermediate A might also show opioid effects. It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9232. The 2014 annual manufacturing quota was 6 grammes.

Dimepheptanol, also known as methadol or racemethadol, is a synthetic opioid analgesic related to methadone. It has similar effects to other opioids, including analgesia, sedation and euphoria, as well as side effects like itching, nausea and respiratory depression.

Alphamethadol (INN), or α-methadol, also known as alfametadol, is a synthetic opioid analgesic. It is an isomer of dimepheptanol (methadol), the other being betamethadol (β-methadol). Alphamethadol is composed of two isomers itself, L-α-methadol, and D-α-methadol. The former compound, L-α-methadol, is an important active metabolite of levacetylmethadol (LAAM), an opioid substitute drug that is used clinically. Both of alphamethadol's isomers bind to and activate the μ-opioid receptor and are active as opioid analgesics, similarly to those of alphacetylmethadol (α-acetylmethadol).

Racemorphan, or morphanol, is the racemic mixture of the two stereoisomers of 17-methylmorphinan-3-ol, each with differing pharmacology and effects:

Isomethadone (INN, BAN; trade name Liden; also known as isoamidone) is a synthetic opioid analgesic and antitussive related to methadone that was used formerly as a pharmaceutical drug but is now no longer marketed. Isomethadone was used as both an analgesic and antitussive. It binds to and activates both the μ- and δ-opioid receptors, with the (S)-isomer being the more potent of the two enantiomers. Isomethadone is a Schedule II controlled substance in the United States, with an ACSCN of 9226 and a 2014 aggregate manufacturing quota of 5 g. The salts in use are the hydrobromide (HBr, free base conversion ratio 0.793), hydrochloride (HCl, 0.894), and HCl monohydrate (0.850). Isomethadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.

Alphacetylmethadol (INN), or α-acetylmethadol (AAM), is a synthetic opioid analgesic. Its levorotary enantiomer, levacetylmethadol, is an FDA-approved treatment for opioid addiction; however as of 2003 it is no longer used in the United States for this purpose. Alphacetylmethadol is very similar in structure to methadone, a widely prescribed treatment for opioid addiction. In the United States, it is a Schedule I controlled substance under the Controlled Substances Act, with an ACSCN of 9603 and a 2013 annual manufacturing quota of 2 grammes.

Noracymethadol (INN) is a synthetic opioid analgesic related to methadone that was never marketed. In a clinical trial of postpartum patients it was reported to produce analgesia comparable to that of morphine but with less nausea, dizziness, and drowsiness. Other side effects included salivation, ataxia, and respiratory depression that was reversible by naloxone. Similarly to many of its analogues, noracymethadol is a Schedule I controlled substance in the United States with an ACSCN of 9633 and 2013 annual manufacturing quota of 12 grammes. and is also controlled internationally under the United Nations Single Convention on Narcotic Drugs of 1961. The salts known are the gluconate and hydrochloride (0.903).




















