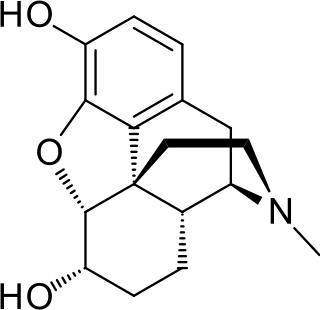
Dihydromorphine is a semi-synthetic opioid structurally related to and derived from morphine. The 7,8-double bond in morphine is reduced to a single bond to get dihydromorphine. Dihydromorphine is a moderately strong analgesic and is used clinically in the treatment of pain and also is an active metabolite of the analgesic opioid drug dihydrocodeine. Dihydromorphine occurs in trace quantities in assays of opium on occasion, as does dihydrocodeine, dihydrothebaine, tetrahydrothebaine, etc. The process for manufacturing dihydromorphine from morphine for pharmaceutical use was developed in Germany in the late 19th century, with the synthesis being published in 1900 and the drug introduced clinically as Paramorfan shortly thereafter. A high-yield synthesis from tetrahydrothebaine was later developed.

Dipipanone (Pipadone) is a strong opioid analgesic drug, used for acute pain by mouth (PO) for adults — initially 10 mg every 6 hours, then increased if necessary up to 30 mg every 6 hours, with the dose to be increased gradually. It is often used in instances where morphine is indicated but cannot be used due to the patient being allergic to morphine. In analgesic potency 25 mg dipipanone is approximately equivalent to 10 mg morphine.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Levorphanol is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.

Levomethorphan (LVM) (INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine.

Methorphan comes in two isomeric forms, each with differing pharmacology and effects:

Lefetamine (Santenol) is a drug which is a stimulant and also an analgesic with effects comparable to codeine.

Thebacon, or dihydrocodeinone enol acetate, is a semisynthetic opioid that is similar to hydrocodone and is most commonly synthesised from thebaine. Thebacon was invented in Germany in 1924, four years after the first synthesis of hydrocodone. Thebacon is a derivative of acetyldihydrocodeine, where only the 6–7 double bond is saturated. Thebacon is marketed as its hydrochloride salt under the trade name Acedicon, and as its bitartrate under Diacodin and other trade names. The hydrochloride salt has a free base conversion ratio of 0.846. Other salts used in research and other settings include thebacon's phosphate, hydrobromide, citrate, hydroiodide, and sulfate.
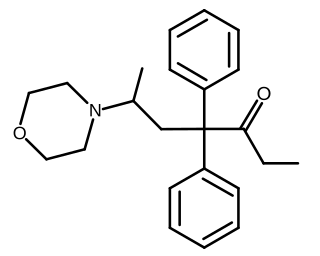
Phenadoxone is an opioid analgesic of the open chain class invented in Germany by Hoechst in 1947. It is one of a handful of useful synthetic analgesics which were used in the United States for various lengths of time in the 20 or so years after the end of the Second World War but which were withdrawn from the market for various or no known reason and which now are mostly in Schedule I of the United States' Controlled Substances Act of 1970, or in Schedule II but not produced or marketed in the US. Others on this list are ketobemidone (Ketogin), dextromoramide, phenazocine, dipipanone, piminodine (Alvodine), propiram (Algeril), anileridine (Leritine) and alphaprodine (Nisentil).

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.
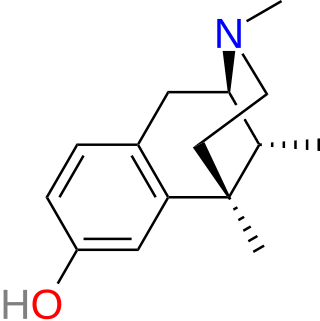
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects, mediated through a mixed agonist–antagonist action at the mu opioid receptor, its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors and/or sigma receptors.

Phenazocine is an opioid analgesic drug, which is related to pentazocine and has a similar profile of effects.

Dimenoxadol (INN), or dimenoxadole (BAN), is an opioid analgesic which is a benzilic acid derivative, closely related to benactyzine. Further, the structure is similar to methadone and related compounds like dextropropoxyphene.

Drotebanol (Oxymethebanol) is a morphinan derivative that acts as an opioid agonist. It was invented by Sankyo Company in Japan during the 1970s. It is synthesised from thebaine.

Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.

Racemoramide, or simply moramide, is an opioid analgesic and a racemic mixture of the substances dextromoramide and levomoramide, two enantiomers of a chiral molecule.

Racemorphan, or morphanol, is the racemic mixture of the two stereoisomers of 17-methylmorphinan-3-ol, each with differing pharmacology and effects:

Alphacetylmethadol (INN), or α-acetylmethadol (AAM), is a synthetic opioid analgesic. Its levorotary enantiomer, levacetylmethadol, is an FDA-approved treatment for opioid addiction; however as of 2003 it is no longer used in the United States for this purpose. Alphacetylmethadol is very similar in structure to methadone, a widely prescribed treatment for opioid addiction. In the United States, it is a Schedule I controlled substance under the Controlled Substances Act, with an ACSCN of 9603 and a 2013 annual manufacturing quota of 2 grammes.

Noracymethadol (INN) is a synthetic opioid analgesic related to methadone that was never marketed. In a clinical trial of postpartum patients it was reported to produce analgesia comparable to that of morphine but with less nausea, dizziness, and drowsiness. Other side effects included salivation, ataxia, and respiratory depression that was reversible by naloxone. Similarly to many of its analogues, noracymethadol is a Schedule I controlled substance in the United States with an ACSCN of 9633 and 2013 annual manufacturing quota of 12 grammes. and is also controlled internationally under the United Nations Single Convention on Narcotic Drugs of 1961. The salts known are the gluconate and hydrochloride (0.903).

Levomethadone, sold under the brand name L-Polamidon among others, is a synthetic opioid analgesic and antitussive which is marketed in Europe and is used for pain management and in opioid maintenance therapy. In addition to being used as a pharmaceutical drug itself, levomethadone is the main therapeutic component of methadone.



















