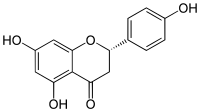
Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
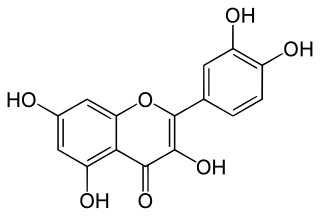
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones are produced almost exclusively by the members of the bean family, Fabaceae (Leguminosae).

Rutin is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid glycoside found in a wide variety of plants, including citrus.

Naringin is a flavanone-7-O-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness (debittering) created by naringin. In humans naringin is metabolized to the aglycone naringenin by naringinase present in the gut.

Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
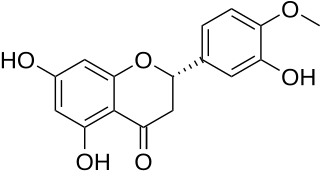
Hesperetin is the 4'-methoxy derivative of eriodictyol, a flavanone. Hesperetin's 7-O-glycoside, hesperidin, is a naturally occurring flavanon-glycoside, the main flavonoid in lemons and sweet oranges. Hesperetin are not found to a significant extent in Citrus spp.
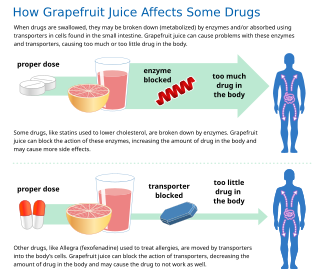
Some fruit juices and fruits can interact with numerous drugs, in many cases causing adverse effects. The effect is most studied with grapefruit and grapefruit juice, but similar effects have been observed with certain other citrus fruits.

Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one).
Naringinase is a debittering enzyme that is used in the commercial production of citrus juices. It breaks down the compound naringin that gives citrus juices its bitter taste. It is a multienzyme complex which possesses alpha-L-rhamnosidase and beta glucosidase active centers. The E.C. No.(EC 3.2.1.40) of the naringinase and rhamnosidase are the same. First rhamnosidase breaks naringin into prunin and rhamnose. Lastly glucosidase breaks prunin into glucose and naringenin, a flavorless flavanone also found in various citrus.
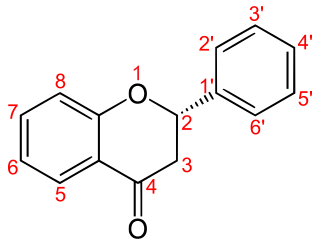
The flavanones, a type of flavonoids, are various aromatic, colorless ketones derived from flavone that often occur in plants as glycosides.
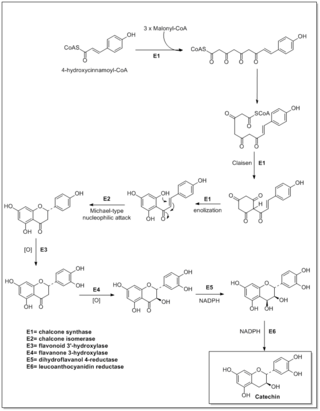
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.
In enzymology, a flavanone 7-O-beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
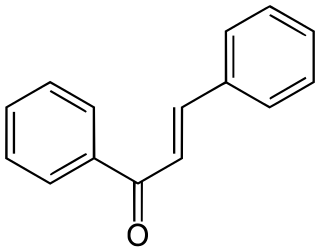
Chalconoids, also known as chalcones, are natural phenols derived from chalcone. They form the central core for a variety of important biological compounds.

Narirutin is a flavanone-7-O-glycoside, consisting of the flavanone naringenin bonded with the disaccharide rutinose.

Melitidin is a flavanone glycoside. Melitidin was discovered in bergamot orange juice and exhibits statin-like properties in preclinical research.
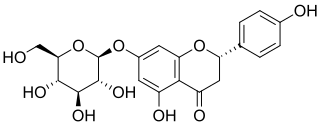
Prunin is a flavanone glycoside found in immature citrus fruits and in tomatoes. Its aglycone form is called naringenin.