
A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.
Desogestrel is a progestin medication which is used in birth control pills. It is also used in the treatment of menopausal symptoms in women. The medication is available and used alone or in combination with an estrogen. It is taken by mouth.
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

Gestrinone, sold under the brand names Dimetrose and Nemestran among others, is a medication which is used in the treatment of endometriosis. It has also been used to treat other conditions such as uterine fibroids and heavy menstrual bleeding and has been investigated as a method of birth control. Gestrinone is used alone and is not formulated in combination with other medications. It is taken by mouth or in through the vagina.

Etynodiol diacetate, or ethynodiol diacetate, sold under the brand name Ovulen among others, is a progestin medication which is used in birth control pills. The medication is available only in combination with an estrogen. It is taken by mouth.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women. It is also used in menopausal hormone therapy. The medication is available almost exclusively in combination with an estrogen. It is taken by mouth.

Lynestrenol, sold under the brand names Exluton and Ministat among others, is a progestin medication which is used in birth control pills and in the treatment of gynecological disorders. The medication is available both alone and in combination with an estrogen. It is taken by mouth.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Trestolone, also known as 7α-methyl-19-nortestosterone (MENT), is an experimental androgen/anabolic steroid (AAS) and progestogen medication which has been under development for potential use as a form of hormonal birth control for men and in androgen replacement therapy for low testosterone levels in men but has never been marketed for medical use. It is given as an implant that is placed into fat. As trestolone acetate, an androgen ester and prodrug of trestolone, the medication can also be given by injection into muscle.

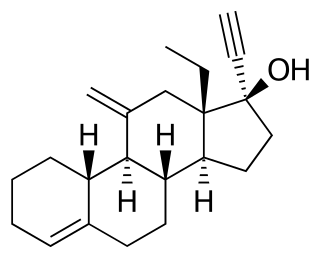
Dienogest, sold under the brand name Visanne among others, is a progestin medication which is used in birth control pills and in the treatment of endometriosis. It is also used in menopausal hormone therapy and to treat heavy periods. Dienogest is available both alone and in combination with estrogens. It is taken by mouth.

Metribolone is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was never marketed for medical use but has been widely used in scientific research as a hot ligand in androgen receptor (AR) ligand binding assays (LBAs) and as a photoaffinity label for the AR. More precisely, metribolone is the 17α-methylated derivative of trenbolone. It was investigated briefly for the treatment of advanced breast cancer in women in the late 1960s and early 1970s, but was found to produce signs of severe hepatotoxicity at very low dosages, and its development was subsequently discontinued.

Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders. It is taken by mouth.

Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed. It is taken by mouth.

Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was available both alone and in combination with an estrogen. The medication is taken by mouth.
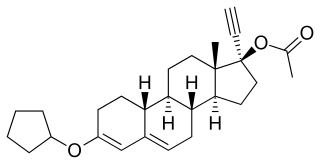
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Norgesterone, also known as norvinodrel or vinylestrenolone and sold under the brand name Vestalin, is a progestin medication which was formerly used in birth control pills for women but is now no longer marketed. It was used in combination with the estrogen ethinylestradiol. It is taken by mouth.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

Dimethyltrienolone is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and 17α-alkylated 19-nortestosterone (nandrolone) derivative which was never marketed for medical use. It has among the highest known affinity of any AAS for the androgen receptors, and has been said to be perhaps the most potent AAS to have ever been developed.




















