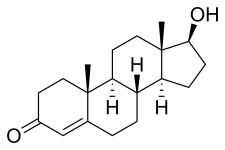
Nandrolone, also known as 19-nortestosterone, is an endogenous androgen which exists in the male body at a ratio of 1:50 compared to testosterone. It is also an anabolic steroid (AAS) which is medically used in the form of esters such as nandrolone decanoate and nandrolone phenylpropionate. Nandrolone esters are used in the treatment of anemias, cachexia, osteoporosis, breast cancer, and for other indications. They are not used by mouth and instead are given by injection into muscle or fat.

Ethylestrenol, also known as ethyloestrenol or ethylnandrol and sold under the brand names Maxibolin and Orabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used in the past for a variety of indications such as to promote weight gain and to treat anemia and osteoporosis but has been discontinued for use in humans. It is still available for veterinary use in Australia and New Zealand however. It is taken by mouth.

Norethandrolone, sold under the brand names Nilevar and Pronabol among others, is an androgen and anabolic steroid (AAS) medication which has been used to promote muscle growth and to treat severe burns, physical trauma, and aplastic anemia but has mostly been discontinued. It is still available for use in France however. It is taken by mouth.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Metribolone is a synthetic and orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was never marketed for medical use but has been widely used in scientific research as a hot ligand in androgen receptor (AR) ligand binding assays (LBAs) and as a photoaffinity label for the AR. More precisely, metribolone is the 17α-methylated derivative of trenbolone. It was investigated briefly for the treatment of advanced breast cancer in women in the late 1960s and early 1970s, but was found to produce signs of severe hepatotoxicity at very low dosages, and its development was subsequently discontinued.

Normethandrone, also known as methylestrenolone or methylnortestosterone and sold under the brand name Metalutin among others, is a progestin and androgen/anabolic steroid (AAS) medication which is used in combination with an estrogen in the treatment of amenorrhea and menopausal symptoms in women. It is taken by mouth.

Altrenogest, sold under the brand names Swinemate and Altren manufactured by Aurora Pharmaceutical and Regumate manufactured by Merck, is a progestin of the 19-nortestosterone group which is widely used in veterinary medicine to suppress or synchronize estrus in horses and pigs. It is available for veterinary use in both Europe and the United States.
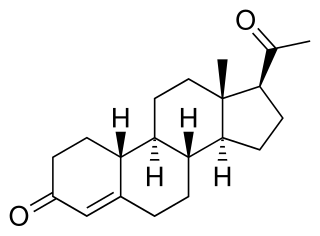
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.

Norvinisterone, sold under the brand names Neoprogestin and Nor-Progestelea, is a progestin and androgen/anabolic steroid (AAS) medication which was used in Europe but is now no longer marketed. It is taken by mouth.

Dimethandrolone (DMA), also known by its developmental code name CDB-1321, is an experimental androgen/anabolic steroid (AAS) and progestogen medication which is under investigation for potential clinical use.
A 17α-alkylated anabolic steroid is a synthetic anabolic–androgenic steroid (AAS) that features an alkyl group, specifically a methyl or ethyl group, at the C17α position. Unlike many other AAS, 17α-alkylated AAS are orally active and do not require intramuscular injection. However, they uniquely possess a high potential for hepatotoxicity, which simultaneously limits their use. In addition, some have a high risk of gynecomastia due to uniquely high estrogenic activity, although this does not apply to 17α-alkylated AAS that are also 4,5α-reduced or 19-demethylated. The prototypical example of a 17α-alkylated AAS is methyltestosterone (17α-methyltestosterone).

Ethyltestosterone, or 17α-ethyltestosterone, also known as 17α-ethylandrost-4-en-17β-ol-3-one or 17α-pregn-4-en-17-ol-3-one, is a synthetic, orally active anabolic–androgenic steroid (AAS) of the 17α-alkylated group related to methyltestosterone which was never marketed. Like methyltestosterone, ethyltestosterone is the parent compound of many AAS. Derivatives of ethyltestosterone include norethandrolone, ethylestrenol (ethylnandrol), norboletone, ethyldienolone, tetrahydrogestrinone, bolenol (ethylnorandrostenol), and propetandrol.

Dimethyltrienolone is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and 17α-alkylated 19-nortestosterone (nandrolone) derivative which was never marketed for medical use. It has among the highest known affinity of any AAS for the androgen receptors, and has been said to be perhaps the most potent AAS to have ever been developed.

Vinyltestosterone is a synthetic anabolic–androgenic steroid (AAS) that was never marketed. However, two 19-nortestosterone derivatives of vinyltestosterone, norvinisterone (17α-vinyl-19-nortestosterone) and norgesterone, have been marketed. They are used as progestins for female hormonal contraception, rather than as AAS.

11β-Methyl-19-nortestosterone (11β-MNT) is a synthetic and orally active anabolic–androgenic steroid (AAS) and a derivative of nandrolone (19-nortestosterone) which was developed by the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development (NICHD) and has not been marketed at this time.

5α-Dihydronorethisterone is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.

17α-Allyl-19-nortestosterone, also known as 3-ketoallylestrenol or as 17α-allylestr-4-en-17β-ol-3-one, is a progestin which was never marketed. It is a combined derivative of the anabolic–androgenic steroid and progestogen nandrolone (19-nortestosterone) and the antiandrogen allyltestosterone (17α-allyltestosterone). The drug is a major active metabolite of allylestrenol, which is thought to be a prodrug of 17α-allyl-19-nortestosterone.

The structure–activity relationships (SAR) of anabolic steroids (AAS) have been extensively studied.