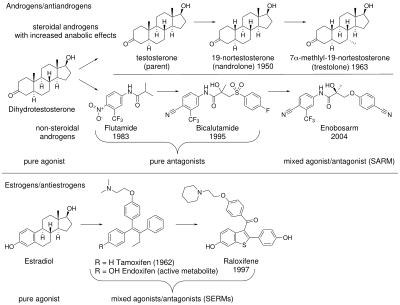
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.
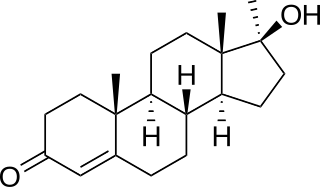
Methyltestosterone, sold under the brand names Android, Metandren, and Testred among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, at low doses as a component of menopausal hormone therapy for menopausal symptoms like hot flashes, osteoporosis, and low sexual desire in women, and to treat breast cancer in women. It is taken by mouth or held in the cheek or under the tongue.

Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are a class of drugs that are structurally related to testosterone, the main male sex hormone, and produce effects by binding to the androgen receptor. Anabolic steroids have a number of medical uses, but are also used by athletes to increase muscle size, strength, and performance.

BMS-564,929 is an investigational selective androgen receptor modulator (SARM) which is being developed by Bristol-Myers Squibb for treatment of the symptoms of age-related decline in androgen levels in men ("andropause"). These symptoms may include depression, loss of muscle mass and strength, reduction in libido and osteoporosis. Treatment with exogenous testosterone is effective in counteracting these symptoms but is associated with a range of side effects, the most serious of which is enlargement of the prostate gland, which can lead to benign prostatic hypertrophy and even prostate cancer. This means there is a clinical need for selective androgen receptor modulators, which produce anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in the prostate.

S-40503 is an investigational selective androgen receptor modulator (SARM) developed by the Japanese company Kaken Pharmaceuticals, which was developed for the treatment of osteoporosis. SARMs are a new class of drugs which produce tissue-specific anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in other tissues such as in the prostate gland, thus avoiding side effects such as benign prostatic hypertrophy which can occur following treatment with unselective androgens like testosterone or anabolic steroids.

LGD-2226 is an investigational selective androgen receptor modulator (SARM), which is being developed for treatment of muscle wasting and osteoporosis.

Enobosarm, also formerly known as ostarine and by the developmental code names GTx-024, MK-2866, and S-22, is a selective androgen receptor modulator (SARM) which is under development for the treatment of androgen receptor-positive breast cancer in women and for improvement of body composition in people taking GLP-1 receptor agonists like semaglutide. It was also under development for a variety of other indications, including treatment of cachexia, Duchenne muscular dystrophy, muscle atrophy or sarcopenia, and stress urinary incontinence, but development for all other uses has been discontinued. Enobosarm was evaluated for the treatment of muscle wasting related to cancer in late-stage clinical trials, and the drug improved lean body mass in these trials, but it was not effective in improving muscle strength. As a result, enobosarm was not approved and development for this use was terminated. Enobosarm is taken by mouth.
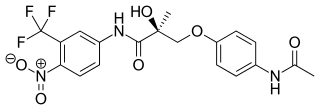
Andarine is a selective androgen receptor modulator (SARM) which was developed by GTX, Inc for the treatment of conditions such as muscle wasting, osteoporosis, and benign prostatic hypertrophy (BPH), using the nonsteroidal antiandrogen bicalutamide as a lead compound. Development of andarine for all indications has been discontinued, in favor of the structurally related and improved compound enobosarm.
The first antiandrogen was discovered in the 1960s. Antiandrogens antagonise the androgen receptor (AR) and thereby block the biological effects of testosterone and dihydrotestosterone (DHT). Antiandrogens are important for men with hormonally responsive diseases like prostate cancer, benign prostatic hyperplasia (BHP), acne, seborrhea, hirsutism and androgen alopecia. Antiandrogens are mainly used for the treatment of prostate diseases. Research from 2010 suggests that ARs could be linked to the disease progression of triple-negative breast cancer and salivary duct carcinoma and that antiandrogens can potentially be used to treat it.
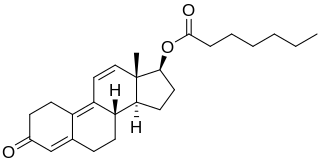
Trenbolone enanthate, known by the nickname Trenabol, is a synthetic and injected anabolic–androgenic steroid (AAS) and a derivative of nandrolone which was never marketed. It is the C17β enanthate ester and a long-acting prodrug of trenbolone. Trenbolone enanthate was never approved for medical or veterinary use but is used in scientific research and has been sold on the internet black market as a designer steroid for bodybuilders and athletes.

LGD-4033, also known by the developmental code name VK5211 and by the black-market name Ligandrol, is a selective androgen receptor modulator (SARM) which is under development for the treatment of muscle atrophy in people with hip fracture. It was also under development for the treatment of cachexia, hypogonadism, and osteoporosis, but development for these indications was discontinued. LGD-4033 has been reported to dose-dependently improve lean body mass and muscle strength in preliminary clinical trials, but is still being developed and has not been approved for medical use. The drug is taken by mouth.

Vosilasarm, also known by the development codes RAD140 and EP0062 and by the black-market name Testolone or Testalone, is a selective androgen receptor modulator (SARM) which is under development for the treatment of hormone-sensitive breast cancer. It is specifically under development for the treatment of androgen receptor-positive, estrogen receptor-negative, HER2-negative advanced breast cancer. Vosilasarm was also previously under development for the treatment of sarcopenia, osteoporosis, and weight loss due to cancer cachexia, but development for these indications was discontinued. The drug is taken by mouth.

LG121071 is a selective androgen receptor modulator (SARM) developed by Ligand Pharmaceuticals that was first described in 1999 and was the first orally active nonsteroidal androgen to be discovered. It is a tricyclic quinolone derivative, structurally distinct from other nonsteroidal AR agonists like andarine and enobosarm (ostarine). The drug acts as a high-affinity full agonist of the androgen receptor (AR), with a potency and efficacy that is said to be equivalent to that of dihydrotestosterone (DHT). Unlike testosterone, but similarly to DHT, LG121071 and other nonsteroidal androgens cannot be potentiated by 5α-reductase in androgenic tissues, and for this reason, show tissue-selective androgenic effects. In accordance, they are said to possess full anabolic activity with reduced androgenic activity, similarly to anabolic-androgenic steroids.

Dimethandrolone (DMA), also known by its developmental code name CDB-1321, is an experimental androgen/anabolic steroid (AAS) and progestogen medication which is under investigation for potential clinical use.
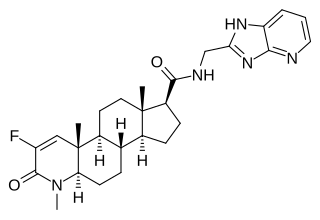
MK-0773, also known as PF-05314882, is a steroidal, orally active selective androgen receptor modulator (SARM) that was under development by Merck and GTx for the treatment of sarcopenia in women and men. Clinical trials for sarcopenia began in late 2007 but the collaboration between Merck and GTx ended in early 2010 and GTx terminated development of MK-0773 shortly thereafter. MK-0773 was developed as a more advanced version of the related compound TFM-4AS-1.

LG-120907 is a nonsteroidal antiandrogen (NSAA) of the quinoline group which was developed by Ligand Pharmaceuticals along with selective androgen receptor modulators (SARMs) like LG-121071 and was never marketed. The drug is a high-affinity antagonist of the androgen receptor (AR) with a Ki value of 26 nM and has been found to inhibit growth of the ventral prostate and seminal vesicles in male rats without increasing circulating levels of luteinizing hormone or testosterone. However, this tissue selectivity has not been assessed in humans. LG-120907 is orally active and shows greater oral potency than the arylpropionamide NSAA flutamide.

The pharmacology of bicalutamide is the study of the pharmacodynamic and pharmacokinetic properties of the nonsteroidal antiandrogen (NSAA) bicalutamide. In terms of pharmacodynamics, bicalutamide acts as a selective antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has no capacity to activate the AR. It does not decrease androgen levels and has no other important hormonal activity. The medication has progonadotropic effects due to its AR antagonist activity and can increase androgen, estrogen, and neurosteroid production and levels. This results in a variety of differences of bicalutamide monotherapy compared to surgical and medical castration, such as indirect estrogenic effects and associated benefits like preservation of sexual function and drawbacks like gynecomastia. Bicalutamide can paradoxically stimulate late-stage prostate cancer due to accumulated mutations in the cancer. When used as a monotherapy, bicalutamide can induce breast development in males due to its estrogenic effects. Unlike other kinds of antiandrogens, it may have less adverse effect on the testes and fertility.

RU-59063 is a nonsteroidal androgen or selective androgen receptor modulator (SARM) which was first described in 1994 and was never marketed. It was originally thought to be a potent antiandrogen, but subsequent research found that it actually possesses dose-dependent androgenic activity, albeit with lower efficacy than dihydrotestosterone (DHT). The drug is an N-substituted arylthiohydantoin and was derived from the first-generation nonsteroidal antiandrogen (NSAA) nilutamide. The second-generation NSAAs enzalutamide, RD-162, and apalutamide were derived from RU-59063.