A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.
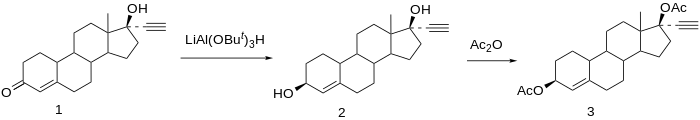
Desogestrel is a progestin medication which is used in birth control pills for women. It is also used in the treatment of menopausal symptoms in women. The medication is available and used alone or in combination with an estrogen. It is taken by mouth.

Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is an analog of the drug spironolactone. Drospirenone is taken by mouth.
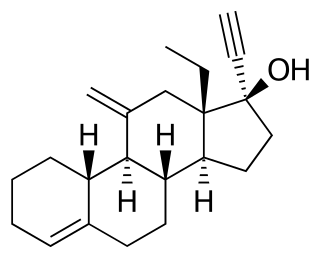
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Norelgestromin, or norelgestromine, sold under the brand names Evra and Ortho Evra among others, is a progestin medication which is used as a method of birth control for women. The medication is available in combination with an estrogen and is not available alone. It is used as a patch that is applied to the skin.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

Norgestrel is a progestin which is used in birth control pills sold under the brand name Ovral in combination with the estrogen ethinylestradiol and Opill by itself. It is also used in menopausal hormone therapy. It is taken by mouth.

Etynodiol, or ethynodiol, is a steroidal progestin of the 19-nortestosterone group which was never marketed. A diacylated derivative, etynodiol diacetate, is used as a hormonal contraceptive. Etynodiol is sometimes used as a synonym for etynodiol diacetate.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women. It is also used in menopausal hormone therapy. The medication is available almost exclusively in combination with an estrogen. It is taken by mouth.

Lynestrenol, sold under the brand names Exluton and Ministat among others, is a progestin medication which is used in birth control pills and in the treatment of gynecological disorders. The medication is available both alone and in combination with an estrogen. It is taken by mouth.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of hormonal birth control which is used to prevent pregnancy in women. It is used both as a form of progestogen-only injectable birth control and in combined injectable birth control formulations. It may be used following childbirth, miscarriage, or abortion. The failure rate per year in preventing pregnancy for the progestogen-only formulation is 2 per 100 women. Each dose of this form lasts two months with only up to two doses typically recommended.

Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was available both alone and in combination with an estrogen. The medication is taken by mouth.
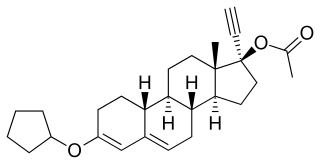
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Ethynerone, also known as 17α-(2-chloroethynyl)estra-4,9-dien-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was first reported in 1961 but was never marketed. Under the developmental code name MK-665, it was studied in combination with mestranol as an oral contraceptive. Development of the drug was discontinued due to concerns surrounding toxicity findings in dogs. It is a chloroethynylated derivative of norethisterone.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Ethinylandrostenediol, also known as 17α-ethynyl-5-androstenediol, is a synthetic estrogen, progestogen, and androgen which was never marketed. It is the C17α ethynyl derivative of the androgen precursor and prohormone 5-androstenediol.