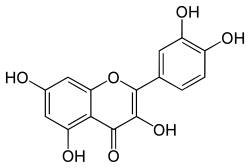
Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Phytochemicals are chemical compounds produced by plants, generally to help them resist fungi, bacteria and plant virus infections, and also consumption by insects and other animals. The name comes from Greek φυτόν (phyton) 'plant'. Some phytochemicals have been used as poisons and others as traditional medicine.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
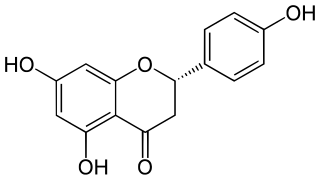
Naringenin is a flavanone from the flavonoid group of polyphenols and is commonly found in a variety of citrus fruits and is the predominant flavonone in grapefruit. Naringenin has demonstrated numerous biological activities, including anti-inflammatory properties, antioxidant activity and skin healing. It is used as a cosmetic ingredient and dietary supplement.

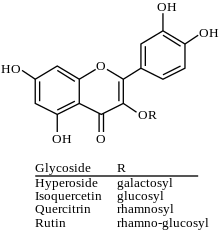
Rutin is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid glycoside found in a wide variety of plants, including citrus.

Quercitrin is a glycoside formed from the flavonoid quercetin and the deoxy sugar rhamnose.

Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol, a type of flavonoid, found in a variety of plants and plant-derived foods including kale, beans, tea, spinach, and broccoli. Kaempferol is a yellow crystalline solid with a melting point of 276–278 °C (529–532 °F). It is slightly soluble in water and highly soluble in hot ethanol, ethers, and DMSO. Kaempferol is named for 17th-century German naturalist Engelbert Kaempfer.
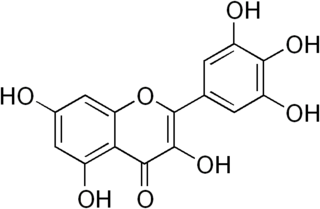
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine.

A polyphenol antioxidant is a hypothetized type of antioxidant, in which each instance would contain a polyphenolic substructure; such instances which have been studied in vitro. Numbering over 4,000 distinct chemical structures, such polyphenols may have antioxidant activity {{{1}}} in vitro (although they are unlikely to be antioxidants in vivo). Hypothetically, they may affect cell-to-cell signaling, receptor sensitivity, inflammatory enzyme activity or gene regulation, although high-quality clinical research has not confirmed any of these possible effects in humans as of 2020.

Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one).

Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.
In enzymology, a quercetin-3,3'-bissulfate 7-sulfotransferase is an enzyme that catalyzes the chemical reaction

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Fisetin (7,3′,4′-flavon-3-ol) is a plant flavonol from the flavonoid group of polyphenols. It can be found in many plants, where it serves as a yellow/ochre colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers. Its chemical formula was first described by Austrian chemist Josef Herzig in 1891.

Isorhamnetin is an O-methylated flavon-ol from the class of flavonoids. A common food source of this 3'-methoxylated derivative of quercetin and its glucoside conjugates are pungent yellow or red onions, in which it is a minor pigment, quercetin-3,4'-diglucoside and quercetin-4'-glucoside and the aglycone quercetin being the major pigments. Pears, olive oil, wine and tomato sauce are rich in isorhamnetin. Almond skin is a rich source of isorhamnetin-3-O-rutinoside and isorhamnetin-3-O-glucoside, in some cultivars they comprise 75% of the polyphenol content, the total of which can exceed 10 mg/100 gram almond. Others sources include the spice, herbal medicinal and psychoactive Mexican tarragon (Tagetes lucida), which is described as accumulating isorhamnetin and its 7-O-glucoside derivate. Nopal is also a good source of isorhamnetin, which can be extracted by supercritical fluid extraction assisted by enzymes.

Miquelianin is a flavonol glucuronide, a type of phenolic compound present in wine, in species of St John's wort, like Hypericum hirsutum, in Nelumbo nucifera or in green beans.

Quercetin 3-sulfate is a plasma human metabolite of quercetin. It is the sulfate conjugate of quercetin.