A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use.

Adenosine (symbol A or Ado) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9-glycosidic bond. Adenosine is one of four nucleoside building blocks to DNA and RNA, which are essential for all life. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP.

The adenosine receptors (or P1 receptors) are a class of purinergic G protein-coupled receptors with adenosine as the endogenous ligand. There are four known types of adenosine receptors in humans: A1, A2A, A2B and A3; each is encoded by a different gene.

Aminophylline is a compound of the bronchodilator theophylline with ethylenediamine in 2:1 ratio. The ethylenediamine improves solubility, and the aminophylline is usually found as a dihydrate.
Sigma receptors (σ-receptors) are protein cell surface receptors that bind ligands such as 4-PPBP, SA 4503 (cutamesine), ditolylguanidine, dimethyltryptamine, and siramesine. There are two subtypes, sigma-1 receptors (σ1) and sigma-2 receptors (σ2), which are classified as sigma receptors for their pharmacological similarities, even though they are evolutionarily unrelated.

The adenosine A1 receptor is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand.

The adenosine A2A receptor, also known as ADORA2A, is an adenosine receptor, and also denotes the human gene encoding it.

The adenosine A3 receptor, also known as ADORA3, is an adenosine receptor, but also denotes the human gene encoding it.

The adenosine A2B receptor, also known as ADORA2B, is a G-protein coupled adenosine receptor, and also denotes the human adenosine A2b receptor gene which encodes it.

8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, PD-116,948) is a drug which acts as a potent and selective antagonist for the adenosine A1 receptor. It has high selectivity for A1 over other adenosine receptor subtypes, but as with other xanthine derivatives DPCPX also acts as a phosphodiesterase inhibitor, and is almost as potent as rolipram at inhibiting PDE4. It has been used to study the function of the adenosine A1 receptor in animals, which has been found to be involved in several important functions such as regulation of breathing and activity in various regions of the brain, and DPCPX has also been shown to produce behavioural effects such as increasing the hallucinogen-appropriate responding produced by the 5-HT2A agonist DOI, and the dopamine release induced by MDMA, as well as having interactions with a range of anticonvulsant drugs.

SCH-58261 is a drug which acts as a potent and selective antagonist for the adenosine receptor A2A, with more than 50x selectivity for A2A over other adenosine receptors. It has been used to investigate the mechanism of action of caffeine, which is a mixed A1 / A2A antagonist, and has shown that the A2A receptor is primarily responsible for the stimulant and ergogenic effects of caffeine, but blockade of both A1 and A2A receptors is required to accurately replicate caffeine's effects in animals. SCH-58261 has also shown antidepressant, nootropic and neuroprotective effects in a variety of animal models, and has been investigated as a possible treatment for Parkinson's disease.
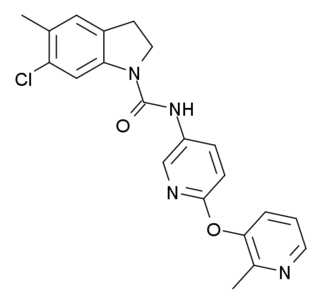
SB-242084 is a psychoactive drug and research chemical which acts as a selective antagonist for the 5HT2C receptor. It has anxiolytic effects, and enhances dopamine signalling in the limbic system, as well as having complex effects on the dopamine release produced by cocaine, increasing it in some brain regions but reducing it in others. It has been shown to increase the effectiveness of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants, and may also reduce their side effects. In animal studies, SB-242084 produced stimulant-type activity and reinforcing effects, somewhat similar to but much weaker than cocaine or amphetamines.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.
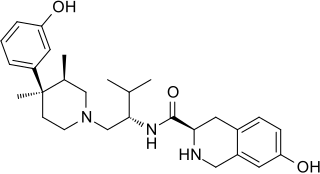
JDTic is a selective, long-acting ("inactivating") antagonist of the κ-opioid receptor (KOR). JDTic is a 4-phenylpiperidine derivative, distantly related structurally to analgesics such as pethidine and ketobemidone, and more closely to the MOR antagonist alvimopan. In addition, it is structurally distinct from other KOR antagonists such as norbinaltorphimine. JDTic has been used to create crystal structures of KOR [ PDB: 4DJH, 6VI4].

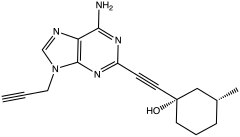
PSB-10 is a drug which acts as a selective antagonist for the adenosine A3 receptor (ki value at human A3 receptor is 0.44 nM), with high selectivity over the other three adenosine receptor subtypes (ki values at human A1, A2A and A2B receptors are 4.1, 3.3 and 30 μM). Further pharmacological experiments in a [35S]GTPγS binding assay using hA3-CHO-cells indicated that PSB-10 acts as an inverse agonist (IC50 = 4 nM). It has been shown to produce antiinflammatory effects in animal studies. Simple xanthine derivatives such as caffeine and DPCPX have generally low affinity for the A3 subtype and must be extended by expanding the ring system and adding an aromatic group to give high A3 affinity and selectivity. The affinity towards adenosine A3 subtype was measured against the radioligand PSB-11.

CGS-15943 is a drug which acts as a potent and reasonably selective antagonist for the adenosine receptors A1 and A2A, having a Ki of 3.3nM at A2A and 21nM at A1. It was one of the first adenosine receptor antagonists discovered that is not a xanthine derivative, instead being a triazoloquinazoline. Consequently, CGS-15943 has the advantage over most xanthine derivatives that it is not a phosphodiesterase inhibitor, and so has more a specific pharmacological effects profile. It produces similar effects to caffeine in animal studies, though with higher potency.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.

Purinergic signalling is a form of extracellular signalling mediated by purine nucleotides and nucleosides such as adenosine and ATP. It involves the activation of purinergic receptors in the cell and/or in nearby cells, thereby regulating cellular functions.

Aticaprant is a selective antagonist of the κ-opioid receptor (KOR) which was originally developed by Eli Lilly and was under development by Janssen Pharmaceuticals for the treatment of major depressive disorder, smoking withdrawal, and other indications. By February 2022, the development of aticaprant was discontinued for all indications.
An adenosine receptor antagonist is a drug which acts as an antagonist of one or more of the adenosine receptors. Examples include caffeine, theophylline, and theobromine.