
A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use.
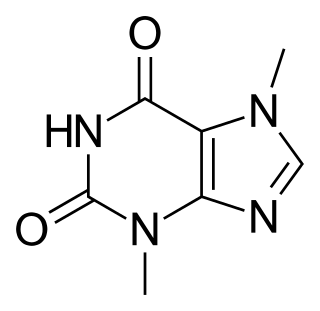
Theobromine, also known as xantheose, is an alkaloid whose name is derived from the Theobroma family. It is the principal alkaloid of Theobroma cacao (cacao plant). It tastes bitter and has the chemical formula C7H8N4O2. It is found in chocolate, as well as in a number of other foods, including the leaves of the tea plant, and the kola nut.

Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones.

Xanthine is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine.

Allopurinol is a medication used to decrease high blood uric acid levels. It is specifically used to prevent gout, prevent specific types of kidney stones and for the high uric acid levels that can occur with chemotherapy. It is taken by mouth or injected into a vein.

The Merck Group, branded and commonly known as Merck, is a German multinational science and technology company headquartered in Darmstadt, with about 57,000 employees and present in 66 countries. The group includes around 250 companies; the main company is Merck KGaA in Germany. The company is divided into three business lines: Healthcare, Life Sciences and Electronics. Merck was founded in 1668 and is the world's oldest operating chemical and pharmaceutical company, as well as one of the largest pharmaceutical companies in the world.

The adenosine receptors (or P1 receptors) are a class of purinergic G protein-coupled receptors with adenosine as the endogenous ligand. There are four known types of adenosine receptors in humans: A1, A2A, A2B and A3; each is encoded by a different gene.
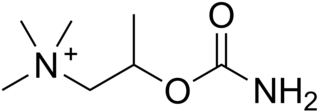
Bethanechol is a parasympathomimetic choline carbamate that selectively stimulates muscarinic receptors without any effect on nicotinic receptors. Unlike acetylcholine, bethanechol is not hydrolyzed by cholinesterase and will therefore have a long duration of action. Bethanechol is sold under the brand names Duvoid (Roberts), Myotonachol (Glenwood), Urecholine and Urocarb (Hamilton). The name bethanechol refers to its structure as the urethane of beta-methylcholine.

Lisinopril is a medication of the angiotensin-converting enzyme (ACE) inhibitor and is used to treat high blood pressure, heart failure, and after heart attacks. For high blood pressure it is usually a first-line treatment. It is also used to prevent kidney problems in people with diabetes mellitus. Lisinopril is taken by mouth. Full effect may take up to four weeks to occur.
The ECA stack is a drug combination used in weight loss and as a stimulant. ECA is an initialism for ephedrine, caffeine, and aspirin, with variants of it including the EC stack, which removes the aspirin for those who can not tolerate it. Dietary supplements based on or including elements of ECA were popular through the 1990s and early 2000s, but the marketing of ephedra- or ephedrine-containing stimulant combinations for weight loss and bodybuilding is now restricted or illegal in the United States, Canada and Netherlands due to reports of heart attack, stroke, and death associated with these supplements.

Cyproheptadine, sold under the brand name Periactin among others, is a first-generation antihistamine with additional anticholinergic, antiserotonergic, and local anesthetic properties.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.

Febuxostat, sold under the brand names Uloric and Adenuric among others, is a medication used long-term to treat gout due to high uric acid levels. It is generally recommended only for people who cannot take allopurinol. When initially started, medications such as NSAIDs are often recommended to prevent gout flares. It is taken by mouth.

8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, PD-116,948) is a drug which acts as a potent and selective antagonist for the adenosine A1 receptor. It has high selectivity for A1 over other adenosine receptor subtypes, but as with other xanthine derivatives DPCPX also acts as a phosphodiesterase inhibitor, and is almost as potent as rolipram at inhibiting PDE4. It has been used to study the function of the adenosine A1 receptor in animals, which has been found to be involved in several important functions such as regulation of breathing and activity in various regions of the brain, and DPCPX has also been shown to produce behavioural effects such as increasing the hallucinogen-appropriate responding produced by the 5-HT2A agonist DOI, and the dopamine release induced by MDMA, as well as having interactions with a range of anticonvulsant drugs.

Laropiprant (INN) was a drug used in combination with niacin to reduce blood cholesterol that is no longer sold, due to increases in side-effects with no cardiovascular benefit. Laropiprant itself has no cholesterol lowering effect, but it reduces facial flushes induced by niacin.
A xanthine oxidase inhibitor is any substance that inhibits the activity of xanthine oxidase, an enzyme involved in purine metabolism. In humans, inhibition of xanthine oxidase reduces the production of uric acid, and several medications that inhibit xanthine oxidase are indicated for treatment of hyperuricemia and related medical conditions including gout. Xanthine oxidase inhibitors are being investigated for management of reperfusion injury.
Dipeptidyl peptidase-4 inhibitors are enzyme inhibitors that inhibit the enzyme dipeptidyl peptidase-4 (DPP-4). They are used in the treatment of type 2 diabetes mellitus. Inhibition of the DPP-4 enzyme prolongs and enhances the activity of incretins that play an important role in insulin secretion and blood glucose control regulation. Type 2 diabetes mellitus is a chronic metabolic disease that results from inability of the β-cells in the pancreas to secrete sufficient amounts of insulin to meet the body's needs. Insulin resistance and increased hepatic glucose production can also play a role by increasing the body's demand for insulin. Current treatments, other than insulin supplementation, are sometimes not sufficient to achieve control and may cause undesirable side effects, such as weight gain and hypoglycemia. In recent years, new drugs have been developed, based on continuing research into the mechanism of insulin production and regulation of the metabolism of sugar in the body. The enzyme DPP-4 has been found to play a significant role.

CGS-15943 is a drug which acts as a potent and reasonably selective antagonist for the adenosine receptors A1 and A2A, having a Ki of 3.3nM at A2A and 21nM at A1. It was one of the first adenosine receptor antagonists discovered that is not a xanthine derivative, instead being a triazoloquinazoline. Consequently, CGS-15943 has the advantage over most xanthine derivatives that it is not a phosphodiesterase inhibitor, and so has more a specific pharmacological effects profile. It produces similar effects to caffeine in animal studies, though with higher potency.
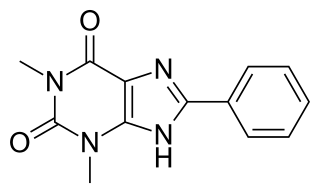
8-Phenyltheophylline (8-phenyl-1,3-dimethylxanthine, 8-PT) is a drug derived from the xanthine family which acts as a potent and selective antagonist for the adenosine receptors A1 and A2A, but unlike other xanthine derivatives has virtually no activity as a phosphodiesterase inhibitor. It has stimulant effects in animals with similar potency to caffeine. Coincidentally 8-phenyltheophylline has also been found to be a potent and selective inhibitor of the liver enzyme CYP1A2 which makes it likely to cause interactions with other drugs which are normally metabolised by CYP1A2.

Merck & Co., Inc. is an American multinational pharmaceutical company headquartered in Rahway, New Jersey. It is named after the Merck family, which set up Merck Group in Germany in 1668. The company does business as Merck Sharp & Dohme (MSD) outside the United States and Canada.

















