An anorectic or anorexic is a drug which reduces appetite, resulting in lower food consumption, leading to weight loss. These substances work by affecting the central nervous system or certain neurotransmitters to create a feeling of fullness or reduce the desire to eat. The understanding of anorexiant effects is crucial in the development of interventions for weight management, eating disorders, and related health concerns. The anorexiant effect can be induced through diverse mechanisms, ranging from hormonal regulation to neural signaling. Ghrelin, leptin, and peptide YY are among the hormones involved in appetite control. Additionally, neurotransmitters such as serotonin and dopamine in the central nervous system contribute significantly to the regulation of food intake.

4-Methylaminorex is a stimulant drug of the 2-amino-5-aryloxazoline class that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a stimulant.

Medazepam is a drug that is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative, and skeletal muscle relaxant properties. It is known by the following brand names: Azepamid, Nobrium, Tranquirax, Rudotel, Raporan, Ansilan and Mezapam. Medazepam is a long-acting benzodiazepine drug. The half-life of medazepam is 36–200 hours.

Aminorex is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension. In the U.S., it is an illegal Schedule I drug, meaning it has high abuse potential, no accepted medical use, and a poor safety profile.

Chlorphentermine is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the 4-chloro derivative of the better known appetite suppressant phentermine, which is still in current use.

Desoxypipradrol, also known as 2-diphenylmethylpiperidine (2-DPMP), is a drug developed by Ciba in the 1950s which acts as a norepinephrine-dopamine reuptake inhibitor (NDRI).

Xylopropamine, also known as 3,4-dimethylamphetamine, is a stimulant drug of the phenethylamine and amphetamine classes which was developed and marketed as an appetite suppressant in the 1950s.
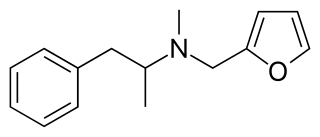
Furfenorex (Frugalan), also known as furfurylmethylamphetamine, is a stimulant drug which was developed in the 1960s and used as an appetite suppressant. It produces methamphetamine as a metabolite, and has been withdrawn from the market due to abuse potential.

(-)-1-Phenyl-2-propylaminopentane is a stimulant of the substituted phenethylamine class that has been derived from selegiline. When compared with selegiline and other substituted phenethylamines (-)-PPAP has a notably different mechanism of action and pharmacological effect.

Pipequaline (INN) is an anxiolytic drug that was never marketed. It possesses a novel chemical structure that is not closely related to other drugs of this type. The drug has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and very little sedative, amnestic or anticonvulsant effects, and so is classified as a nonbenzodiazepine anxiolytic.
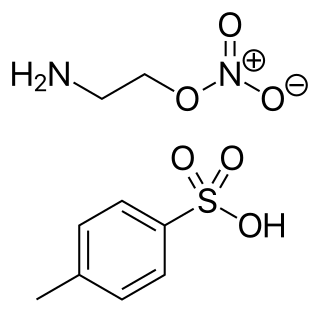
Itramin tosilate (INN), or itramin tosylate, is a vasodilator.
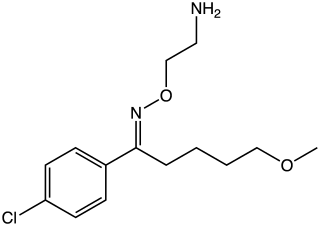
Clovoxamine (INN) is a drug that was discovered in the 1970s and was subsequently investigated as an antidepressant and anxiolytic agent but was never marketed. It acts as a serotonin-norepinephrine reuptake inhibitor (SNRI), with little affinity for the muscarinic acetylcholine, histamine, adrenergic, and serotonin receptors. The compound is structurally related to fluvoxamine.

Ciclazindol (WY-23409) is an antidepressant and anorectic drug of the tetracyclic chemical class that was developed in the mid to late 1970s, but was never marketed. It acts as a norepinephrine reuptake inhibitor, and to a lesser extent as a dopamine reuptake inhibitor. Ciclazindol has no effects on the SERT, 5-HT receptors, mACh receptors, or α-adrenergic receptors, and has only weak affinity for the H1 receptor. As suggested by its local anesthetic properties, ciclazindol may also inhibit sodium channels. It is known to block potassium channels as well.

Indeloxazine (INN) is an antidepressant and cerebral activator that was marketed in Japan and South Korea by Yamanouchi Pharmaceutical Co., Ltd for the treatment of psychiatric symptoms associated with cerebrovascular diseases, namely depression resulting from stroke, emotional disturbance, and avolition. It was marketed from 1988 to 1998, when it was removed from the market reportedly for lack of effectiveness.

RDS-127 is a drug which is used in scientific research. It acts as a D2-like receptor agonist and also has some serotonin and adrenergic agonist effects, as well as some anticholinergic action, and produces both anorectic and pro-sexual effects in animal studies.

A norepinephrine–dopamine reuptake inhibitor (NDRI) is a drug used for the treatment of clinical depression, attention deficit hyperactivity disorder (ADHD), narcolepsy, and the management of Parkinson's disease. The drug acts as a reuptake inhibitor for the neurotransmitters norepinephrine and dopamine by blocking the action of the norepinephrine transporter (NET) and the dopamine transporter (DAT), respectively. This in turn leads to increased extracellular concentrations of both norepinephrine and dopamine and, therefore, an increase in adrenergic and dopaminergic neurotransmission.
Hydroxyethylrutosides are hydroxyethyl derivatives of rutosides. Examples include:

Tolufazepam is a drug that is a benzodiazepine derivative. Studies have shown tolufazepam to have anticonvulsant and anxiolytic activity in animal subjects, including convulsions elicited by pentylenetetrazol.

Piperoxan, also known as benodaine, was the first antihistamine to be discovered. This compound, derived from benzodioxan, was prepared in the early 1930s by Daniel Bovet and Ernest Fourneau at the Pasteur Institute in France. Formerly investigated by Fourneau as an α-adrenergic-blocking agent, they demonstrated that it also antagonized histamine-induced bronchospasm in guinea pigs, and published their findings in 1933. Bovet went on to win the 1957 Nobel Prize in Physiology or Medicine for his contribution. One of Bovet and Fourneau's students, Anne-Marie Staub, published the first structure–activity relationship (SAR) study of antihistamines in 1939. Piperoxan and analogues themselves were not clinically useful due to the production of toxic effects in humans and were followed by phenbenzamine (Antergan) in the early 1940s, which was the first antihistamine to be marketed for medical use.

3-Chloromethamphetamine is a substituted amphetamine derivative invented in the 1960s. In animal studies it was deemed to be a "hallucinogen" rather than a stimulant, though the assays used at the time did not distinguish between the compounds now termed psychedelics and those now termed empathogens.



















