
An analgesic drug, also called simply an analgesic, pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.
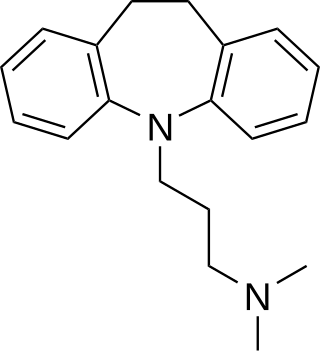
Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants, which is important for the management of depression. They are second-line drugs next to SSRIs and SNRIs. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms.
Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.

Tramadol, sold under the brand name Ultram among others, is an opioid pain medication and a serotonin–norepinephrine reuptake inhibitor (SNR) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen).

Venlafaxine, sold under the brand name Effexor among others, is an antidepressant medication of the serotonin-norepinephrine reuptake inhibitor (SNRI) class. It is used to treat major depressive disorder, generalized anxiety disorder, panic disorder, and social anxiety disorder. Studies have shown that Venlafaxine improves quality of life. It may also be used for chronic pain. It is taken by mouth. It is also available as the salt venlafaxine besylate in an extended-release formulation.

Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a synthetic opioid pain medication of the phenylpiperidine class. Synthesized in 1938 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, Germany. Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines, the prodines, bemidones and others more distant, including diphenoxylate and analogues.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, obsessive–compulsive disorder (OCD), social phobia, attention-deficit hyperactivity disorder (ADHD), chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.
DOV Pharmaceutical was a biotechnology company that focused on therapies primarily for central nervous system conditions. It was founded in 1995 by former employees of American Cyanamid, and in 1998 it in-licensed drugs discovered at American Cyanamid for further development. It held an IPO on NASDQ in 2002, and its shares plunged days later after negative details about a past relationship between Élan and DOV emerged.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

Desmetramadol, also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.

A serotonin reuptake inhibitor (SRI) is a type of drug which acts as a reuptake inhibitor of the neurotransmitter serotonin by blocking the action of the serotonin transporter (SERT). This in turn leads to increased extracellular concentrations of serotonin and, therefore, an increase in serotonergic neurotransmission. It is a type of monoamine reuptake inhibitor (MRI); other types of MRIs include dopamine reuptake inhibitors and norepinephrine reuptake inhibitors.
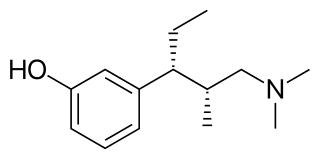
Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration, and lasts for 4–6 hours.
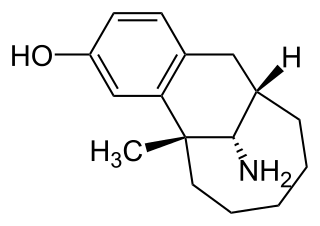
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.

Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, that is primarily used to treat moderate to severe pain.
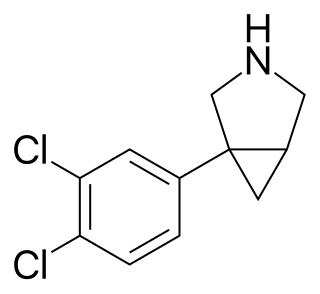
DOV 216,303 is an experimental antidepressant drug originally developed by DOV Pharmaceutical and was licensed to Merck & Co. in 2004; Merck and DOV terminated their relationship in December 2006.
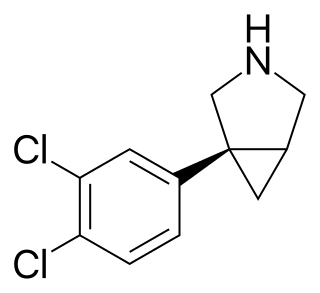
DOV-102,677 is a psychoactive drug being developed by Merck and is currently in clinical trials. It is a triple reuptake inhibitor (TRI), or serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI). It is the (−)-enantiomer of DOV-216,303, and its (+)-enantiomer is DOV-21,947 (amitifadine).

Amitifadine is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) or so-called triple reuptake inhibitor (TRI) which is or was being developed by Euthymics Bioscience It was under development for the treatment of major depressive disorder, but in May 2013, it was reported that the drug failed to show superior efficacy to placebo in a phase IIb/IIIa clinical trial. It was suggested that this may have been due to the drug being underdosed. In September 2017, development of amitifadine for the treatment of major depressive disorder was finally officially discontinued. As of September 2017, it is still listed as being under development for the treatment of alcoholism and smoking withdrawal.

A serotonin–dopamine reuptake inhibitor (SDRI) is a type of drug which acts as a reuptake inhibitor of the monoamine neurotransmitters serotonin and dopamine by blocking the actions of the serotonin transporter (SERT) and dopamine transporter (DAT), respectively. This in turn leads to increased extracellular concentrations of serotonin and dopamine, and, therefore, an increase in serotonergic and dopaminergic neurotransmission.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

Centanafadine (INN) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) that began its development with Euthymics Bioscience after they acquired DOV Pharmaceutical. It was developed as a treatment for attention-deficit hyperactivity disorder (ADHD) and inhibits the reuptake of norepinephrine, dopamine, and serotonin with a ratio of 1:6:14, respectively. In 2011, Euthymics Bioscience spun off its development of centanafadine to a new company called Neurovance. In March 2017, Otsuka Pharmaceutical acquired Neurovance and the rights to centanafadine. As of January 2018, Otsuka's pipeline indicates it is in Phase II and III clinical trials for a number of different applications to medical conditions.
















