Trifluoperazine, marketed under the brand name Stelazine among others, is a typical antipsychotic primarily used to treat schizophrenia. It may also be used short term in those with generalized anxiety disorder but is less preferred to benzodiazepines. It is of the phenothiazine chemical class. It was approved for medical use in the United States in 1959.

Haloperidol, sold under the brand name Haldol among others, is a typical antipsychotic medication. Haloperidol is used in the treatment of schizophrenia, tics in Tourette syndrome, mania in bipolar disorder, delirium, agitation, acute psychosis, and hallucinations from alcohol withdrawal. It may be used by mouth or injection into a muscle or a vein. Haloperidol typically works within 30 to 60 minutes. A long-acting formulation may be used as an injection every four weeks by people with schizophrenia or related illnesses, who either forget or refuse to take the medication by mouth.

Typical antipsychotics are a class of antipsychotic drugs first developed in the 1950s and used to treat psychosis. Typical antipsychotics may also be used for the treatment of acute mania, agitation, and other conditions. The first typical antipsychotics to come into medical use were the phenothiazines, namely chlorpromazine which was discovered serendipitously. Another prominent grouping of antipsychotics are the butyrophenones, an example of which is haloperidol. The newer, second-generation antipsychotics, also known as atypical antipsychotics, have largely supplanted the use of typical antipsychotics as first-line agents due to the higher risk of movement disorders in the latter.

Quetiapine, sold under the brand name Seroquel among others, is an atypical antipsychotic medication used for the treatment of schizophrenia, bipolar disorder, and major depressive disorder. Despite being widely used as a sleep aid due to its sedating effect, the benefits of such use may not outweigh its undesirable side effects. It is taken orally.
Tardive dyskinesia (TD) is a disorder that results in involuntary repetitive body movements, which may include grimacing, sticking out the tongue or smacking the lips. Additionally, there may be chorea or slow writhing movements. In about 20% of people with TD, the disorder interferes with daily functioning. If TD is present in the setting of a long-term drug therapy, reversibility can be determined primarily by severity of symptoms and how long symptoms have been present before the long-term drug has been stopped.
Dyskinesia refers to a category of movement disorders that are characterized by involuntary muscle movements, including movements similar to tics or chorea and diminished voluntary movements. Dyskinesia can be anything from a slight tremor of the hands to an uncontrollable movement of the upper body or lower extremities. Discoordination can also occur internally especially with the respiratory muscles and it often goes unrecognized. Dyskinesia is a symptom of several medical disorders that are distinguished by their underlying cause.
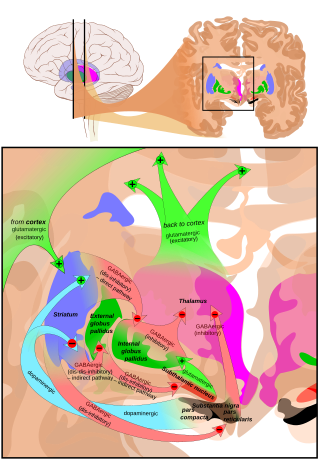
Hyperkinesia refers to an increase in muscular activity that can result in excessive abnormal movements, excessive normal movements, or a combination of both. Hyperkinesia is a state of excessive restlessness which is featured in a large variety of disorders that affect the ability to control motor movement, such as Huntington's disease. It is the opposite of hypokinesia, which refers to decreased bodily movement, as commonly manifested in Parkinson's disease.
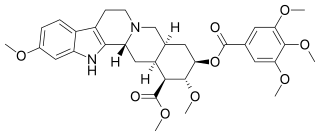
Reserpine is a drug that is used for the treatment of high blood pressure, usually in combination with a thiazide diuretic or vasodilator. Large clinical trials have shown that combined treatment with reserpine plus a thiazide diuretic reduces mortality of people with hypertension. Although the use of reserpine as a solo drug has declined since it was first approved by the FDA in 1955, the combined use of reserpine and a thiazide diuretic or vasodilator is still recommended in patients who do not achieve adequate lowering of blood pressure with first-line drug treatment alone. The reserpine-hydrochlorothiazide combo pill was the 17th most commonly prescribed of the 43 combination antihypertensive pills available In 2012.

Tetrabenazine is a drug for the symptomatic treatment of hyperkinetic movement disorders. It is sold under the brand names Nitoman and Xenazine among others. On August 15, 2008, the U.S. Food and Drug Administration approved the use of tetrabenazine to treat chorea associated with Huntington's disease. Although other drugs had been used "off label," tetrabenazine was the first approved treatment for Huntington's disease in the U.S. The compound has been known since the 1950s.

Amisulpride is an antiemetic and antipsychotic medication used at lower doses intravenously to prevent and treat postoperative nausea and vomiting; and at higher doses by mouth to treat schizophrenia and acute psychotic episodes. It is sold under the brand names Barhemsys and Solian, Socian, Deniban and others. At very low doses it is also used to treat dysthymia.
Extrapyramidal symptoms (EPS) are symptoms that are archetypically associated with the extrapyramidal system of the brain's cerebral cortex. When such symptoms are caused by medications or other drugs, they are also known as extrapyramidal side effects (EPSE). The symptoms can be acute (short-term) or chronic (long-term). They include movement dysfunction such as dystonia, akathisia, parkinsonism characteristic symptoms such as rigidity, bradykinesia, tremor, and tardive dyskinesia. Extrapyramidal symptoms are a reason why subjects drop out of clinical trials of antipsychotics; of the 213 (14.6%) subjects that dropped out of one of the largest clinical trials of antipsychotics, 58 (27.2%) of those discontinuations were due to EPS.

Asenapine, sold under the brand name Saphris among others, is an atypical antipsychotic medication used to treat schizophrenia and acute mania associated with bipolar disorder as well as the medium to long-term management of bipolar disorder.
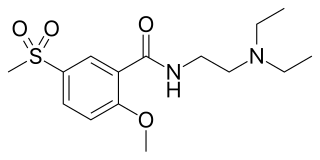
Tiapride is a drug that selectively blocks D2 and D3 dopamine receptors in the brain. It is used to treat a variety of neurological and psychiatric disorders including dyskinesia, alcohol withdrawal syndrome, negative symptoms of psychosis, and agitation and aggression in the elderly. A derivative of benzamide, tiapride is chemically and functionally similar to other benzamide antipsychotics such as sulpiride and amisulpride known for their dopamine antagonist effects.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis and is also being studied for the treatment of Alzheimer's disease psychosis, schizophrenia, agitation, and major depressive disorder. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist.

PBT2 is a safe-for-human-use Zinc ionophore and an experimental drug candidate. It is a second-generation 8-hydroxyquinoline analog intended to be a successor to clioquinol and a potential treatment of Alzheimer's disease and Huntington's disease.

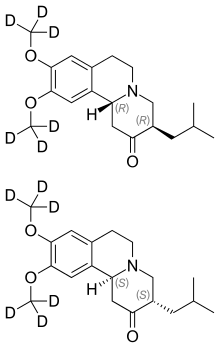
A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms contained in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life.
Risankizumab, sold under the brand name Skyrizi, is a humanized monoclonal antibody used for the treatment of plaque psoriasis, psoriatic arthritis, and Crohn's disease. It is designed to target interleukin 23A (IL-23A). It is given by subcutaneous injection.
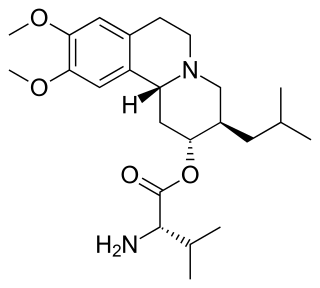
Valbenazine, sold under the brand name Ingrezza, is a medication used to treat tardive dyskinesia. It acts as a vesicular monoamine transporter 2 (VMAT2) inhibitor.
Deulinoleate ethyl is an experimental, orally-bioavailable synthetic deuterated polyunsaturated fatty acid (PUFA), a part of reinforced lipids. It is an isotopologue of linoleic acid, an essential omega-6 PUFA. The deuterated compound, while identical to natural linoleic acid except for the presence of deuterium, is resistant to lipid peroxidation which makes studies of its cell-protective properties worthwhile.

Reinforced lipids are lipid molecules in which some of the fatty acids contain deuterium. They can be used for the protection of living cells by slowing the chain reaction due to isotope effect on lipid peroxidation. The lipid bilayer of the cell and organelle membranes contain polyunsaturated fatty acids (PUFA) are key components of cell and organelle membranes. Any process that either increases oxidation of PUFAs or hinders their ability to be replaced can lead to serious disease. Correspondingly, use of reinforced lipids that stop the chain reaction of lipid peroxidation has preventive and therapeutic potential.