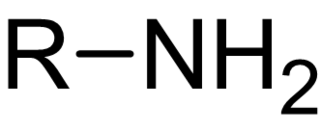
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation (Δ = −327 kcal/mol.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
The Leuckart reaction is the chemical reaction that converts aldehydes or ketones to amines by reductive amination in the presence of heat. The reaction, named after Rudolf Leuckart, uses either ammonium formate or formamide as the nitrogen donor and reducing agent. It requires high temperatures, usually between 120 and 130 °C; for the formamide variant, the temperature can be greater than 165 °C.
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.

Larry E. Overman is Distinguished Professor of Chemistry at the University of California, Irvine. He was born in Chicago in 1943. Overman obtained a B.A. degree from Earlham College in 1965, and he completed his Ph.D. in chemistry from the University of Wisconsin–Madison in 1969, under Howard Whitlock Jr. Professor Overman is a member of the United States National Academy of Sciences and the American Academy of Arts and Sciences. He was the recipient of the Arthur C. Cope Award in 2003, and he was awarded the Tetrahedron Prize for Creativity in Organic Chemistry for 2008.
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.
1-Naphthol, or α-naphthol, is a organic compound with the formula C10H7OH. It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

JNJ-7925476 is a triple reuptake inhibitor antidepressant discovered by Johnson & Johnson, but never marketed.

RTI(-4229)-274, or 2β-( methyl)-3α-(4-fluorophenyl)nortropane is a phenyltropane homologue of paroxetine developed by the group led by F Ivy Carroll in the 1990s.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal M with anionic bis(trimethylsilyl)amide ligands (the −N 2 monovalent anion, or −N 2 monovalent group, and are part of a broader category of metal amides.

3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.















