A beta-lactam (β-lactam) ring is a four-membered lactam. A lactam is a cyclic amide, and beta-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described.

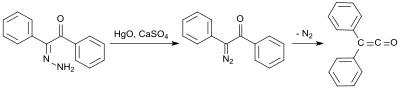
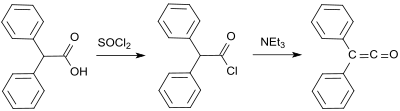
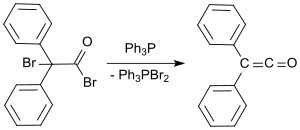
In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation.
The von Richter reaction, also named von Richter rearrangement, is a name reaction in the organic chemistry. It is named after Victor von Richter, who discovered this reaction in year 1871. It is the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol to give the product of cine substitution by a carboxyl group. Although it is not generally synthetically useful due to the low chemical yield and formation of numerous side products, its mechanism was of considerable interest, eluding chemists for almost 100 years before the currently accepted one was proposed.

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. Named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978), the method entails treating an acid chlorides with diazomethane. It is a popular method of producing β-amino acids from α-amino acids.
The Stollé synthesis is a series of chemical reactions that produce oxindoles from anilines and α-haloacid chlorides.

The Finkelstein reaction, named after the German chemist Hans Finkelstein, is an SN2 reaction that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the reaction can be driven to completion by exploiting the differential solubility of halide salts, or by using a large excess of the halide salt.

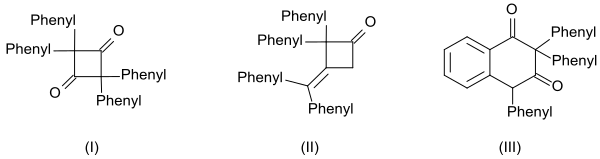
The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.

The Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone. This reaction was first reported by Karl von Auwers in 1908.

The Scholl reaction is a coupling reaction between two arene compounds with the aid of a Lewis acid and a protic acid. It is named after its discoverer, Roland Scholl, a Swiss chemist.
The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.
The Hinsberg oxindole synthesis is a method of preparing oxindoles from the bisulfite additions of glyoxal. It is named after its inventor Oscar Hinsberg.
The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with the Hinsberg reagent in the presence of aqueous alkali. A primary amine will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt. In this way the reaction can distinguish between the three types of amines.
The Kauffmann olefination is a chemical reaction to convert aldehydes and ketones to olefins with a terminal methylene group. This reaction was discovered by the German chemist Thomas Kauffmann and is related to the better known Tebbe olefination or Wittig reaction.
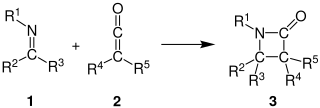
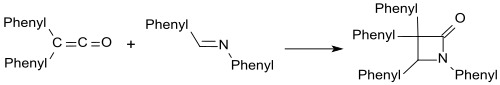
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.
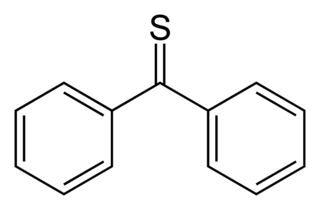
Thiobenzophenone is an organosulfur compound with the formula (C6H5)2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air back to benzophenone and sulfur. Thiobenzophenone is deep blue and dissolves readily in many organic solvents.

1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor. It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.
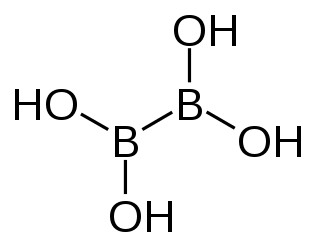
Tetrahydroxydiboron is a chemical reagent which can be used to prepare boronic acids.

3-Dimethylaminoacrolein is an organic compound with the formula Me2NC(H)=CHCHO. It is a pale yellow water-soluble liquid. The compound has a number of useful and unusual properties, e.g. it "causes a reversal of the hypnotic effect of morphine in mice" and has a "stimulating effect in humans".