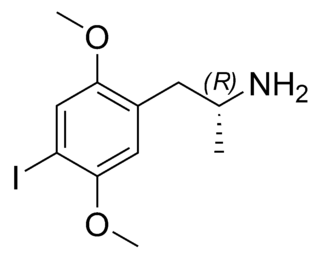
2,5-Dimethoxy-4-iodoamphetamine (DOI) is a psychedelic drug and a substituted amphetamine. Unlike many other substituted amphetamines, however, it is not primarily a stimulant. DOI has a stereocenter and R-(−)-DOI is the more active stereoisomer. In neuroscience research, [125I]-R-(−)-DOI is used as a radioligand and indicator of the presence of 5-HT2A serotonin receptors. DOI's effects have been compared to LSD, although there are differences that experienced users can distinguish. Besides the longer duration, the trip tends to be more energetic than an LSD trip, with more body load and a different subjective visual experience. The after effects include residual stimulation and difficulty sleeping, which, depending on the dose, may persist for days. While rare, it is sometimes sold as a substitute for LSD, or even sold falsely as LSD, which may be dangerous because DOI does not have the same established safety profile as LSD.
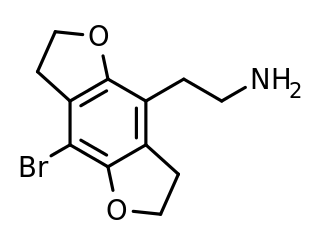
2C-B-FLY is a psychedelic phenethylamine of the 2C family. It was first synthesized in 1996 by Aaron P. Monte.

5-(2-Aminopropyl)-2,3-dihydro-1H-indene (5-APDI), also known as indanylaminopropane (IAP), IAP (psychedelic), 2-API(2-aminopropylindane), indanametamine, and, incorrectly, as indanylamphetamine, is an entactogen and psychedelic drug of the amphetamine family. It has been sold by online vendors through the Internet and has been encountered as a designer drug since 2003, but its popularity and availability has diminished in recent years.

2,5-Dimethoxy-4-trifluoromethylamphetamine (DOTFM) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized in 1994 by a team at Purdue University led by David E. Nichols. DOTFM is the alpha-methylated analogue of 2C-TFM, and is around twice as potent in animal studies. It acts as an agonist at the 5HT2A and 5HT2C receptors. In drug-substitution experiments in rats, DOTFM fully substituted for LSD and was slightly more potent than DOI.

5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is an entactogen and psychedelic designer drug of the amphetamine class. It is a ring-methylated homologue of MDA and a structural isomer of MDMA.

5-(2-Aminopropyl)-2,3-dihydrobenzofuran is a putative entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 3-position oxygen from the 3,4-methylenedioxy ring has been replaced by a methylene bridge. 6-APDB is an analogue of 5-APDB where the 4-position oxygen has been replaced by a methylene bridge instead. 5-APDB was developed by a team led by David E. Nichols at Purdue University as part of their research into non-neurotoxic analogues of MDMA.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.

3-Methoxy-4-methylamphetamine (MMA) is an entactogen and psychedelic drug of the phenethylamine and amphetamine classes. It was first synthesized in 1970 and was encountered as a street drug in Italy in the same decade. MMA was largely forgotten until being reassayed by David E. Nichols as a non-neurotoxic MDMA analogue in 1991, and has subsequently been sold as a designer drug on the internet since the late 2000s (decade).

2-Aminoindane (2-AI) is a research chemical with applications in neurologic disorders and psychotherapy that has also been sold as a designer drug. It acts as a selective substrate for NET and DAT.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.

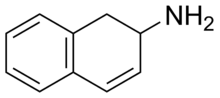
2-Aminotetralin (2-AT), also known as 1,2,3,4-tetrahydronaphthalen-2-amine (THN), is a stimulant drug with a chemical structure consisting of a tetralin group combined with an amine.

6,7-Methylenedioxy-2-aminotetralin (MDAT) is a drug developed in the 1990s by a team at Purdue University led by David E. Nichols. It appears to act as a serotonin releasing agent based on rodent drug discrimination assays comparing it to MDMA, in which it fully substitutes for, and additionally lacks any kind of serotonergic neurotoxicity. Hence, MDAT is considered likely to be a non-neurotoxic, putative entactogen in humans.

6-Chloro-2-aminotetralin (6-CAT) is a drug which acts as a selective serotonin releasing agent (SSRA) and is a putative entactogen in humans. It is a rigid analogue of para-chloroamphetamine (PCA).

2,5-Dimethoxy-4-fluoroamphetamine (DOF) is a psychedelic drug of the phenethylamine and amphetamine classes. Alexander Shulgin briefly describes DOF in his book PiHKAL:
Animal studies that have compared DOF to the highly potent DOI and DOB imply that the human activity will be some four to six times less than these two heavier halide analogues.

6-(2-Aminopropyl)-2,3-dihydrobenzofuran is a stimulant and entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 4-position oxygen from the 3,4-methylenedioxy ring has been replaced with a methylene bridge. 5-APDB (3-Desoxy-MDA) is an analogue of 6-APDB where the 3-position oxygen has been replaced with a methylene instead. 6-APDB, along with 5-APDB, was first synthesized by David E. Nichols in the early 1990s while investigating non-neurotoxic MDMA analogues.

6-Methyl-3,4-methylenedioxyamphetamine (6-Methyl-MDA) is an entactogen and psychedelic drug of the amphetamine class. It was first synthesized in the late 1990s by a team including David E. Nichols at Purdue University while investigating derivatives of 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxy-N-methylamphetamine (MDMA).

6-(2-Aminopropyl)tetralin (6-APT), also sometimes called tetralinylaminopropane (TAP), is a drug of the amphetamine class which acts as a selective serotonin releasing agent (SSRA). It has IC50 values of 121 nM, 6,436 nM, and 3,371 nM for inhibiting the reuptake of serotonin, dopamine, and norepinephrine, respectively. Though it possesses an appreciable in vitro profile, in animal drug discrimination studies it was not found to substitute for MMAI or amphetamine and to only partially substitute for MBDB. This parallels Alexander Shulgin's finding that EDMA (the 1,4-benzodioxine analogue of 6-APT) is inactive, and appears to indicate that the pharmacokinetics of both EDMA and 6-APT may not be favorable.

Difluoromethylenedioxyamphetamine (DiFMDA) is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by Daniel Trachsel and coworkers, along with the corresponding fluorinated derivatives of MDMA, MDEA, BDB and MBDB, with the aim of finding a non-neurotoxic drug able to be used as a less harmful substitute for entactogenic drugs such as MDMA. Since a major route of the normal metabolism of these compounds is scission of the methylenedioxy ring, producing neurotoxic metabolites such as alpha-methyldopamine, it was hoped that the difluoromethylenedioxy bioisostere would show increased metabolic stability and less toxicity.
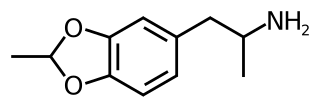
3,4-Ethylidenedioxyamphetamine (EIDA) is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by David Nichols and coworkers, in the course of research to determine the bulk tolerance around the benzodioxole portion of the MDA molecule. EIDA was found to produce similar effects to MDA in animals but with less than half the potency, while the isopropylidenedioxy derivative did not substitute for MDA and instead had sedative and convulsant effects. This shows limited bulk tolerance at this position and makes it likely the activity of EIDA will reside primarily in one enantiomer, although only the racemic mix has been studied as yet.
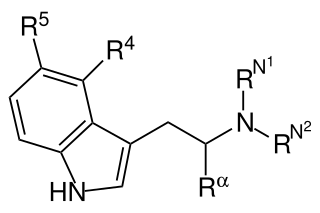
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.