
3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.

David Earl Nichols is an American pharmacologist and medicinal chemist. Previously the Robert C. and Charlotte P. Anderson Distinguished Chair in Pharmacology at Purdue University, Nichols has worked in the field of psychoactive drugs since 1969. While still a graduate student, he patented the method that is used to make the optical isomers of hallucinogenic amphetamines. His contributions include the synthesis and reporting of escaline, LSZ, 6-APB, 2C-I-NBOMe and other NBOMe variants, and several others, as well as the coining of the term "entactogen".
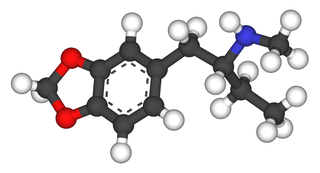
1,3-Benzodioxolyl-N-methylbutanamine (N-methyl-1,3-benzodioxolylbutanamine, MBDB, 3,4-methylenedioxy-N-methyl-α-ethylphenylethylamine) is an entactogen of the phenethylamine chemical class. It is known by the street names Eden and Methyl-J. MBDB is a ring substituted amphetamine and an analogue of MDMA. Like MDMA, it has a methylene dioxy substitution at the 3 and 4 position on the aromatic ring; this is perhaps the most distinctive feature that structurally define analogues of MDMA, in addition to their unique effects, and as a class they are often referred to as "entactogens" to differentiate between typical psychostimulant amphetamines that (as a general rule) are not ring substituted. MBDB differs from MDMA by having an ethyl group instead of a methyl group attached to the alpha carbon; all other parts are identical. Modification at the alpha carbon is uncommon for substituted amphetamines. It has IC50 values of 784 nM against 5-HT, 7825 nM against dopamine, and 1233 nM against norepinephrine. Its metabolism has been described in scientific literature.
2C-BFLY is a psychedelic phenethylamine and designer drug of the 2C family. It was first synthesized in 1996 by Aaron Monte, Professor of Chemistry at UW-La Crosse.

5-(2-Aminopropyl)-2,3-dihydro-1H-indene (5-APDI), also known as indanylaminopropane (IAP), IAP (psychedelic), 2-API(2-aminopropylindane), indanametamine, and, incorrectly, as indanylamphetamine, is an entactogen and psychedelic drug of the amphetamine family. It has been sold by online vendors through the Internet and has been encountered as a designer drug since 2003, but its popularity and availability has diminished in recent years.

The substituted methylenedioxyphenethylamines represent a diverse chemical class of compounds derived from phenethylamines. This category encompasses numerous psychoactive substances with entactogenic, psychedelic, and/or stimulant properties, in addition to entheogens. These compounds find application as research chemicals, designer drugs, and recreational substances.

5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is an entactogen and psychedelic designer drug of the amphetamine class. It is a ring-methylated homologue of MDA and a structural isomer of MDMA.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.

3-Methoxy-4-methylamphetamine (MMA) is an entactogen and psychedelic drug of the phenethylamine and amphetamine classes. It was first synthesized in 1970 and was encountered as a street drug in Italy in the same decade. MMA was largely forgotten until being reassayed by David E. Nichols as a non-neurotoxic MDMA analogue in 1991, and has subsequently been sold as a designer drug on the internet since the late 2000s (decade).

5-Iodo-2-aminoindane (5-IAI) is a drug which acts as a releasing agent of serotonin, norepinephrine, and dopamine. It was developed in the 1990s by a team led by David E. Nichols at Purdue University. 5-IAI fully substitutes for MDMA in rodents and is a putative entactogen in humans. Unlike related aminoindane derivatives like MDAI and MMAI, 5-IAI causes some serotonergic neurotoxicity in rats, but is substantially less toxic than its corresponding amphetamine homologue pIA, with the damage observed barely reaching statistical significance.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.
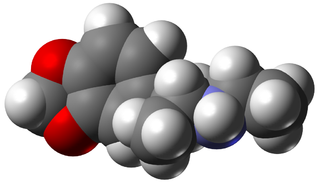
3,4-Methylenedioxy-N-ethylamphetamine is an empathogenic psychoactive drug. MDEA is a substituted amphetamine and a substituted methylenedioxyphenethylamine. MDEA acts as a serotonin, norepinephrine, and dopamine releasing agent and reuptake inhibitor.

6,7-Methylenedioxy-2-aminotetralin (MDAT) is a drug developed in the 1990s by a team at Purdue University led by David E. Nichols. It appears to act as a serotonin releasing agent based on rodent drug discrimination assays comparing it to MDMA, in which it fully substitutes for, and additionally lacks any kind of serotonergic neurotoxicity. Hence, MDAT is considered likely to be a non-neurotoxic, putative entactogen in humans.

6-(2-Aminopropyl)-2,3-dihydrobenzofuran is a stimulant and entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 4-position oxygen from the 3,4-methylenedioxy ring has been replaced with a methylene bridge. 5-APDB (3-Desoxy-MDA) is an analogue of 6-APDB where the 3-position oxygen has been replaced with a methylene instead. 6-APDB, along with 5-APDB, was first synthesized by David E. Nichols in the early 1990s while investigating non-neurotoxic MDMA analogues.

6-Methyl-3,4-methylenedioxyamphetamine (6-Methyl-MDA) is an entactogen and psychedelic drug of the amphetamine class. It was first synthesized in the late 1990s by a team including David E. Nichols at Purdue University while investigating derivatives of 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxy-N-methylamphetamine (MDMA).
3,4-Ethylidenedioxyamphetamine (EIDA) is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by David Nichols and coworkers, in the course of research to determine the bulk tolerance around the benzodioxole portion of the MDA molecule. EIDA was found to produce similar effects to MDA in animals but with less than half the potency, while the isopropylidenedioxy derivative did not substitute for MDA and instead had sedative and convulsant effects. This shows limited bulk tolerance at this position and makes it likely the activity of EIDA will reside primarily in one enantiomer, although only the racemic mix has been studied as yet.

5-MAPDB (1-(2,3-dihydrobenzofuran-5-yl)-N-methylpropan-2-amine) is a chemical compound which acts as an entactogenic drug. It is structurally related to drugs like 5-APDB and 5-MAPB, which have similar effects to MDMA and have been used as recreational drugs. 5-MAPDB has been studied to determine its pharmacological activity, and was found to be a relatively selective serotonin releaser, though with weaker actions as a releaser of other monoamines and 5-HT2 receptor family agonist, similar to older compounds such as 5-APDB.

6-MAPDB is a chemical compound which might be an entactogenic drug. It is structurally related to drugs like 6-APDB and 6-MAPB, which have similar effects to MDMA and have been used as recreational drugs. 6-MAPDB has never been studied to determine its pharmacological activity, though it is the N-methyl derivative of 6-APDB which is known to be a selective serotonin releaser.
25B-NBOH is a derivative of the phenethylamine derived hallucinogen 2C-B which has been sold as a designer drug. It acts as a potent serotonin receptor agonist with similar affinity to the better-known compound 25B-NBOMe at 5-HT2A and 5-HT2C receptors with pKis values of 8.3 and 9.4, respectively.

The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached, and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name Benzofury.



















