
(–)-2-β-Carbomethoxy-3-β-(4-fluorophenyl)tropane is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.

Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. Although RTI holds a strong position in this field, they are not the only researchers that have prepared these analogues. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
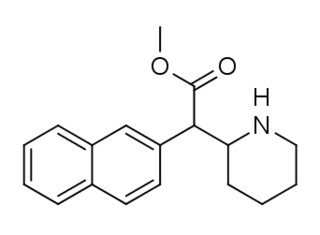
HDMP-28 or methylnaphthidate is a piperidine based stimulant drug, closely related to methylphenidate, but with the benzene ring replaced by naphthalene. It is a potent dopamine reuptake inhibitor, with several times the potency of methylphenidate and a short duration of action, and is a structural isomer of another potent dopamine reuptake inhibitor, N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate.

RTI-126 is a phenyltropane derivative which acts as a potent monoamine reuptake inhibitor and stimulant drug, and has been sold as a designer drug. It is around 5 times more potent than cocaine at inhibiting monoamine reuptake in vitro, but is relatively unselective. It binds to all three monoamine transporters, although still with some selectivity for the dopamine transporter. RTI-126 has a fast onset of effects and short duration of action, and its pharmacological profile in animals is among the closest to cocaine itself out of all the drugs in the RTI series. Its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with its rapid entry into the brain thought to be a key factor in producing its high propensity for development of dependence in animals.
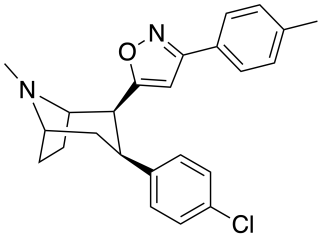
RTI(-4229)-336, is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine, however it produces relatively mild stimulant effects, with a slow onset and long duration of action. These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse. RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration, and significantly reduces rates of cocaine use, especially when combined with SSRIs, and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.
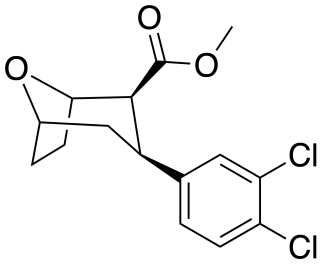
Tropoxane (O-1072) is an aryloxytropane derivative drug developed by Organix Inc., which acts as a stimulant and potent dopamine and serotonin reuptake inhibitor. It is an analogue of dichloropane where the amine nitrogen has been replaced by an oxygen ether link, demonstrating that the amine nitrogen is not required for DAT binding and reuptake inhibition.
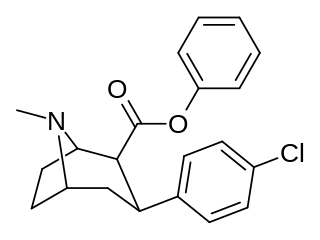
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.

RTI(-4229)-112 is a synthetic stimulant drug from the phenyltropane family. In contrast to RTI-113, which is DAT selective, RTI-112 is a nonselective triple reuptake inhibitor.

RTI(-4229)-177 is a synthetic stimulant drug from the phenyltropane family, which acts as a DRI with micromolar affinity for the SERT. RTI-177 has an unusually long duration of action of 20 hours or more, substantially longer than the related compound RTI-336 from which it differs in molecular structure only by the absence of a p-methyl group.

(–)-2β-Carbomethoxy-3β-(4'-chlorophenyl)tropane (RTI-4229-31) is a synthetic analog of cocaine that acts as a stimulant. Semi-synthesis of this compound is dependent upon the availability of cocaine starting material. According to the article, RTI-31 is 64 x the strength of cocaine in terms of its potency to elicit self-administration in monkeys. WIN 35428 was 6 x weaker than RTI-31, whereas RTI-51 was 2.6 x weaker than RTI-31.

(–)-2β-Carbomethoxy-3β-(4-bromophenyl)tropane is a semi-synthetic alkaloid in the phenyltropane group of psychostimulant compounds. First publicized in the 1990s, it has not been used enough to have gained a fully established profile. RTI-51 can be expected to have properties lying somewhere in between RTI-31 and RTI-55. Importantly it has a ratio of monoamine reuptake inhibition of D > S > N which is an unusual balance of effects not produced by other commonly used compounds. It has been used in its 76Br radiolabelled form to map the distribution of dopamine transporters in the brain.
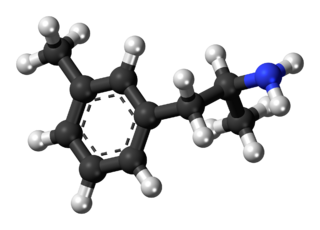
3-Methylamphetamine is a stimulant drug from the amphetamine family. It is self-administered by mice to a similar extent to 4-fluoroamphetamine and has comparable properties as a monoamine releaser, although with a more balanced release of all three monoamines, as opposed to the more dopamine/noradrenaline selective fluoro analogues.
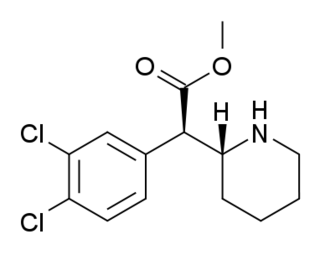
3,4-Dichloromethylphenidate is a stimulant drug related to methylphenidate. Dichloromethylphenidate is a potent psychostimulant that acts as both a dopamine reuptake inhibitor and norepinephrine reuptake inhibitor, meaning it effectively boosts the levels of the norepinephrine and dopamine neurotransmitters in the brain, by binding to, and partially blocking the transporter proteins that normally remove those monoamines from the synaptic cleft.
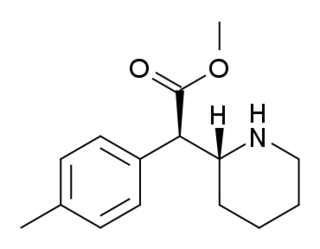
threo-4-Methylmethylphenidate (4-MeTMP) is a stimulant drug related to methylphenidate. It is slightly less potent than methylphenidate and has relatively low efficacy at blocking dopamine reuptake despite its high binding affinity, which led to its investigation as a possible substitute drug for treatment of stimulant abuse. On the other hand, several other simple ring-substituted derivatives of threo-methylphenidate such as the 4-fluoro and 3-chloro compounds are more potent than methylphenidate both in efficacy as dopamine reuptake inhibitors and in animal drug discrimination assays.
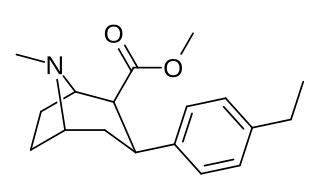
RTI-83 is a phenyltropane derivative which represents a rare example of an SDRI or serotonin-dopamine reuptake inhibitor, a drug which inhibits the reuptake of the neurotransmitters serotonin and dopamine, while having little or no effect on the reuptake of the related neurotransmitter noradrenaline. With a binding affinity (Ki) of 55 nM at DAT and 28.4 nM at SERT but only 4030 nM at NET, RTI-83 has reasonable selectivity for DAT/SERT over NET
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

4-Fluoromethylphenidate is a stimulant drug that acts as a higher potency dopamine reuptake inhibitor than the closely related methylphenidate.


















