The Takeda Pharmaceutical Company Limited is a Japanese multinational pharmaceutical company. It is the third largest pharmaceutical company in Asia, behind Sinopharm and Shanghai Pharmaceuticals, and one of the top 20 largest pharmaceutical companies in the world by revenue. The company has over 49,578 employees worldwide and achieved US$19.299 billion in revenue during the 2018 fiscal year. The company is focused on oncology, rare diseases, neuroscience, gastroenterology, plasma-derived therapies and vaccines. Its headquarters is located in Chuo-ku, Osaka, and it has an office in Nihonbashi, Chuo, Tokyo. In January 2012, Fortune Magazine ranked the Takeda Oncology Company as one of the 100 best companies to work for in the United States. As of 2015, Christophe Weber was appointed as the CEO and president of Takeda.

Erowid, also called Erowid Center, is a non-profit educational organization that provides information about psychoactive plants and chemicals.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.
Salvia divinorum, a psychoactive plant, is legal in most countries. Exceptions, countries where there is some form of control, include Australia, Belgium, Brazil, Canada, Denmark, Estonia, Finland, Germany, Iceland, Ireland, Italy, Japan, South Korea, Norway, Poland, United Kingdom, Ukraine, Spain, Sweden, Armenia and 33 states and territories of the United States.

Bruker Corporation is an American manufacturer of scientific instruments for molecular and materials research, as well as for industrial and applied analysis. It is headquartered in Billerica, Massachusetts, and is the publicly traded parent company of Bruker Scientific Instruments and Bruker Energy & Supercon Technologies (BEST) divisions.

Salvia divinorum is a plant species with transient psychoactive properties when its leaves, or extracts made from the leaves, are administered by smoking, chewing, or drinking. The leaves contain the potent compound salvinorin A and can induce a dissociative state and hallucinations.

Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production was discontinued in June 2017.

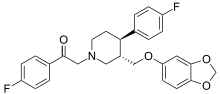
Prinaberel is a synthetic, nonsteroidal, and highly selective agonist of the ERβ subtype of the estrogen receptor. It is used in scientific research to elucidate the role of the ERβ receptor. Studies have indicated that selective ERβ agonists like prinaberel could be useful in the clinical treatment of a variety of medical conditions including inflammatory bowel disease, rheumatoid arthritis, endometriosis, and sepsis. Accordingly, prinaberel either was or still is under investigation by Wyeth for the treatment of some of these conditions.
Calico Life Sciences LLC is a subsidiary of Alphabet Inc. and with a focus on biotechnology their goal is to increase the understanding of the biology that controls human aging, attempting to devise interventions that may enable people to lead longer and healthier lives.

Arbutus Biopharma Corporation is a publicly traded Canadian biopharmaceutical company with an expertise in liposomal drug delivery and RNA interference, and is developing drugs for hepatitis B infection.

Cericlamine is a potent and moderately selective serotonin reuptake inhibitor (SSRI) of the amphetamine family that was investigated as an antidepressant for the treatment of depression, anxiety disorders, and anorexia nervosa by Jouveinal but did not complete development and was never marketed. It reached phase III clinical trials in 1996 before development was discontinued in 1999.