
Sertraline, sold under the brand name Zoloft among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. The effectiveness of sertraline for depression is similar to that of other antidepressants, and the differences are mostly confined to side effects. Sertraline is better tolerated than the older tricyclic antidepressants. Sertraline is effective for panic disorder, social anxiety disorder, generalized anxiety disorder (GAD), and obsessive–compulsive disorder (OCD). Although approved for post-traumatic stress disorder (PTSD), sertraline leads to only modest improvement in this condition. Sertraline also alleviates the symptoms of premenstrual dysphoric disorder (PMDD) and can be used in sub-therapeutic doses or intermittently for its treatment.

Bupropion, sold under the brand name Wellbutrin among others, is an atypical antidepressant primarily used to treat major depressive disorder and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction, it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion, particularly the immediate release formulation, carries a higher risk of seizure than many other antidepressants, hence caution is recommended in patients with a history of seizure disorder.

Venlafaxine, sold under the brand name Effexor among others, is an antidepressant medication of the serotonin–norepinephrine reuptake inhibitor (SNRI) class. It is used to treat major depressive disorder, generalized anxiety disorder, panic disorder, and social anxiety disorder. Studies have shown that venlafaxine improves post-traumatic stress disorder (PTSD). It may also be used for chronic pain. It is taken by mouth. It is also available as the salt venlafaxine besylate in an extended-release formulation.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and migraine prevention. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

Buspirone, sold under the brand name Buspar, among others, is an anxiolytic, a medication primarily used to treat anxiety disorders, particularly generalized anxiety disorder. It is a serotonin 5-HT1A receptor agonist, increasing action at serotonin receptors in the brain. It is taken orally, and takes two to six weeks to be fully effective.

Viloxazine, sold under the brand name Qelbree and formerly as Vivalan among others, is a selective norepinephrine reuptake inhibitor medication which is used in the treatment of attention deficit hyperactivity disorder (ADHD) in children and adults. It was marketed for almost 30 years as an antidepressant for the treatment of depression before being discontinued and subsequently repurposed as a treatment for ADHD. Viloxazine is taken orally. It was used as an antidepressant in an immediate-release form and is used in ADHD in an extended-release form.

Trazodone, sold under many brand names, is an antidepressant medication. It is used to treat major depressive disorder, anxiety disorders, and insomnia. The medication is taken orally.

Nefazodone, sold formerly under the brand names Serzone, Dutonin, and Nefadar among others, is an atypical antidepressant medication which is used in the treatment of depression and for other uses. Nefazodone is still available in the United States, but was withdrawn from other countries due to rare liver toxicity. The medication is taken by mouth.
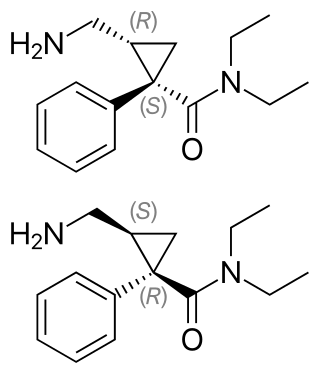
Milnacipran is a serotonin–norepinephrine reuptake inhibitor (SNRI) used in the clinical treatment of fibromyalgia. It is not approved for the clinical treatment of major depressive disorder in the US, but it is in other countries.

Tandospirone, sold under the brand name Sediel, is an anxiolytic and antidepressant medication used in Japan and China, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone class of drugs and is closely related to other azapirones like buspirone and gepirone.

Vilazodone, sold under the brand name Viibryd among others, is a medication used to treat major depressive disorder. It is classified as a serotonin modulator and is taken by mouth.
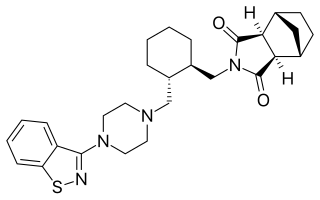
Lurasidone, sold under the brand name Latuda among others, is an antipsychotic medication used to treat schizophrenia and bipolar disorder. It is taken by mouth.

Eptapirone (F-11,440) is a very potent and highly selective 5-HT1A receptor full agonist of the azapirone family. Its affinity for the 5-HT1A receptor was reported to be 4.8 nM (Ki), and its intrinsic activity approximately equal to that of serotonin.

Vortioxetine, sold under the brand names Trintellix and Brintellix among others, is a medication used to treat major depressive disorder. Its effectiveness is viewed as similar to that of other antidepressants. It is taken by mouth.
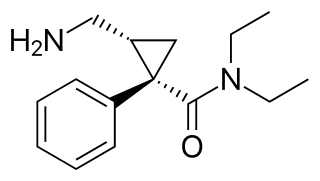
Levomilnacipran is an antidepressant which was approved in the United States in 2013 for the treatment of major depressive disorder (MDD) in adults. It is the levorotatory enantiomer of milnacipran, and has similar effects and pharmacology, acting as a serotonin–norepinephrine reuptake inhibitor (SNRI).
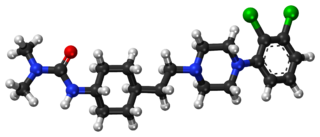
Cariprazine, sold under the brand names Vraylar,Reagila and Symvenu among others, is an atypical antipsychotic developed by Gedeon Richter, which is used in the treatment of schizophrenia, bipolar mania, bipolar depression, and major depressive disorder. It acts primarily as a D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors, with high selectivity for the D3 receptor. It is taken by mouth.

Brexpiprazole, sold under the brand name Rexulti among others, is a medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease. It is an atypical antipsychotic.
TGBA01AD (also known as FKB01MD) is a serotonin reuptake inhibitor, 5-HT1A and 5-HT1D receptor agonist, and 5-HT2 receptor antagonist which is under development by Fabre-Kramer for the treatment of major depressive disorder. It has been in phase II clinical trials since 2009, and as of January 2016, remains in this phase of development.

Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD). Its active components are dextromethorphan (DXM) and bupropion. Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial. It is taken as a tablet by mouth.



















