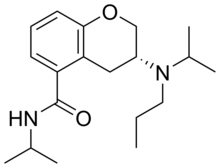
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.
Physical chemistry is the study of macroscopic and particulate phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria.
Quantum chemistry, also called molecular quantum mechanics, is a branch of chemistry focused on the application of quantum mechanics to chemical systems. Understanding electronic structure and molecular dynamics using the Schrödinger equations are central topics in quantum chemistry.
Atomic, molecular, and optical physics (AMO) is the study of matter-matter and light-matter interactions; at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theory includes classical, semi-classical and quantum treatments. Typically, the theory and applications of emission, absorption, scattering of electromagnetic radiation (light) from excited atoms and molecules, analysis of spectroscopy, generation of lasers and masers, and the optical properties of matter in general, fall into these categories.
A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature of chemistry.
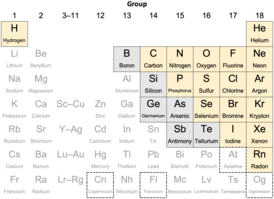
In chemistry, a nonmetal is a type of chemical element generally characterized by low density, low strength, and a tendency—when they react with other elements of this kind—to form acidic compounds. Nonmetals have a metallic, colorless or colored appearance, with about half being solid and half gaseous; in contrast, nearly all metals are silvery-gray solids. Most nonmetals are poor to moderate conductors of heat and electricity, unlike metals. Their reactivity, akin to that of the metals, ranges from highly active to noble ; a difference between metals and nonmetals is that some of the more noble nonmetals are effectively inert.

Peter William Atkins is an English chemist and a Fellow of Lincoln College at the University of Oxford. He retired in 2007. He is a prolific writer of popular chemistry textbooks, including Physical Chemistry, Inorganic Chemistry, and Molecular Quantum Mechanics. Atkins is also the author of a number of popular science books, including Atkins' Molecules, Galileo's Finger: The Ten Great Ideas of Science and On Being.
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction.

A sulfonyl group can refer either to a functional group found primarily in sulfones or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group similarly to acyl groups. Sulfonyl groups can be written as having the general formula R−S(=O)2−R′, where there are two double bonds between the sulfur and oxygen.

Tellurium dioxide (TeO2) is a solid oxide of tellurium. It is encountered in two different forms, the yellow orthorhombic mineral tellurite, β-TeO2, and the synthetic, colourless tetragonal (paratellurite), α-TeO2. Most of the information regarding reaction chemistry has been obtained in studies involving paratellurite, α-TeO2.
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.

The Davy Medal is awarded by the Royal Society of London "for an outstandingly important recent discovery in any branch of chemistry". Named after Humphry Davy, the medal is awarded with a monetary gift, initially of £1000.
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents.

Tetraxenonogold(II), gold tetraxenide(II) or AuXe2+
4 is a cationic complex with a square planar configuration of atoms. It is found in the compound AuXe2+
4(Sb
2F−
11)
2, which exists in triclinic and tetragonal crystal modifications. The AuXe2+
4 ion is stabilised by interactions with the fluoride atoms of the counterion. The Au−Xe bond length is 274 pm (2.74 Å).

Holmium(III) chloride is the inorganic compound with the formula HoCl3. It is a common salt but is mainly used in research. It exhibits the same color-changing behavior seen in holmium oxide, being a yellow in natural lighting and a bright pink color in fluorescent lighting.
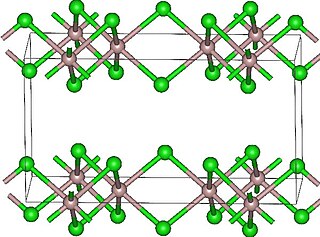
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals and also a hydroscopic hexahydrate LuCl3·6H2O. Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.
John Nogrady was an American male tennis player and coach who was active in the 1940s and 1950s and twice reached the final of the U.S. Pro Tennis Championships.

The metallic elements in the periodic table located between the transition metals and the chemically weak nonmetallic metalloids have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals; none have been recommended by IUPAC. The most common name, post-transition metals, is generally used in this article. Depending on where the adjacent sets of transition metals and metalloids are judged to begin and end, there are at least five competing proposals for which elements to count as post-transition metals: the three most common contain six, ten and thirteen elements, respectively. All proposals include gallium, indium, tin, thallium, lead, and bismuth.

Heavy metals are generally defined as metals with relatively high densities, atomic weights, or atomic numbers. The criteria used, and whether metalloids are included, vary depending on the author and context. In metallurgy, for example, a heavy metal may be defined on the basis of density, whereas in physics the distinguishing criterion might be atomic number, while a chemist would likely be more concerned with chemical behaviour. More specific definitions have been published, but none of these have been widely accepted. The definitions surveyed in this article encompass up to 96 out of the 118 known chemical elements; only mercury, lead and bismuth meet all of them. Despite this lack of agreement, the term is widely used in science. A density of more than 5 g/cm3 is sometimes quoted as a commonly used criterion and is used in the body of this article.