
Pergolide, sold under the brand name Permax and Prascend (veterinary) among others, is an ergoline-based dopamine receptor agonist used in some countries for the treatment of Parkinson's disease. Parkinson's disease is associated with reduced dopamine activity in the substantia nigra of the brain. Pergolide acts on many of the same receptors as dopamine to increase receptor activity.

Chlorphenamine, also known as chlorpheniramine, is an antihistamine used to treat the symptoms of allergic conditions such as allergic rhinitis. It is taken orally. The medication takes effect within two hours and lasts for about 4-6 hours.

Amides of lysergic acid are collectively known as lysergamides, and include a number of compounds with potent agonist and/or antagonist activity at various serotonin and dopamine receptors.
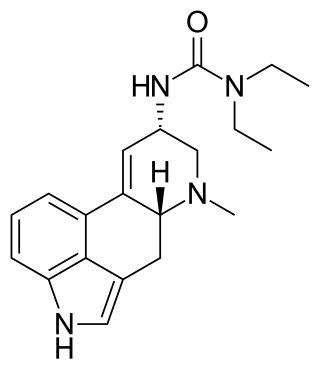
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.
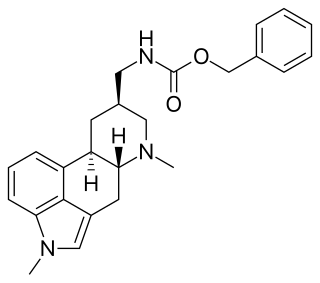
Metergoline, also known as methergoline and sold under the brand names Contralac (veterinary) and Liserdol (clinical), is a monoaminergic medication of the ergoline group which is used as a prolactin inhibitor in the treatment of hyperprolactinemia and to suppress lactation.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.

Zatosetron (LY-277,359) is a drug which acts as an antagonist at the 5HT3 receptor It is orally active and has a long duration of action, producing antinauseant effects but without stimulating the rate of gastrointestinal transport. It is also an effective anxiolytic in both animal studies and human trials, although with some side effects at higher doses.

Xylamidine is a drug which acts as an antagonist at the 5HT2A receptor, and to a lesser extent at the 5HT1A receptor. It does not cross the blood–brain barrier, which makes it useful for blocking peripheral serotonergic responses like cardiovascular and gastrointestinal effects, without producing the central effects of 5HT2A blockade such as sedation, or interfering with the central actions of 5HT2A agonists.
Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

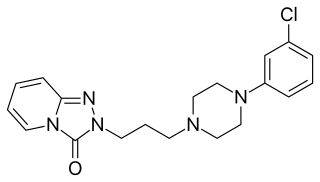
Mepiprazole is an anxiolytic drug of the phenylpiperazine group with additional antidepressant properties that is marketed in Spain. It acts as a 5-HT2A and α1-adrenergic receptor antagonist and inhibits the reuptake and induces the release of serotonin, dopamine, and norepinephrine to varying extents, and has been described as a serotonin antagonist and reuptake inhibitor (SARI). Controlled clinical trials of mepiprazole in patients with irritable bowel syndrome (IBS) were also carried out and suggested some benefits of the drug in relieving symptoms of IBS in some patients. Similarly to other phenylpiperazines like trazodone, nefazodone, and etoperidone, mepiprazole produces mCPP as an active metabolite.
LY-293284 is a research chemical developed by the pharmaceutical company Eli Lilly and used for scientific studies. It acts as a potent and selective 5-HT1A receptor full agonist. It was derived through structural simplification of the ergoline based psychedelic LSD, but is far more selective for 5-HT1A with over 1000x selectivity over other serotonin receptor subtypes and other targets. It has anxiogenic effects in animal studies.

3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.

LY-215,840 is an ergoline derivative drug developed by Eli Lilly, which acts as a potent and selective antagonist at the serotonin 5-HT2 and 5-HT7 receptors. It has anti-hypertensive and muscle relaxant effects in animal studies.

Triazoledione is a phenylpiperazine compound and a major metabolite of the antidepressant nefazodone. It is active, but with substantially reduced potency compared to nefazodone. As such, it has been suggested that it is unlikely that triazoledione contributes significantly to the pharmacology of nefazodone. However, triazoledione may reach concentrations as great as 10 times those of nefazodone, and hence could still be a significant contributor to its therapeutic effects.

Amesergide is a serotonin receptor antagonist of the ergoline and lysergamide families related to methysergide which was under development by Eli Lilly and Company for the treatment of a variety of conditions including depression, anxiety, schizophrenia, male sexual dysfunction, migraine, and thrombosis but was never marketed. It reached phase II clinical trials for the treatment of depression, erectile dysfunction, and premature ejaculation prior to the discontinuation of its development.

AC-90179 is a piperidine derivative which acts as an inverse agonist at the 5-HT2A serotonin receptor and an antagonist at 5-HT2C. It was developed as a potential antipsychotic but was not pursued for medical applications due to poor oral bioavailability; however, it continues to be used as a tool compound in pharmacological research.




















