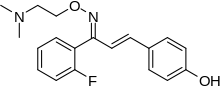
Mirtazapine, sold under the brand name Remeron amongst others, is an atypical antidepressant, and as such is used primarily to treat depression. Its effects may take up to four weeks, but can also manifest as early as one to two weeks. It is often used in cases of depression complicated by anxiety or insomnia. The effectiveness of Mirtazapine is comparable to other commonly prescribed antidepressants. It is taken by mouth.
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug that was first approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was the first drug approved in that class.

Eszopiclone, sold under the brand-name Lunesta among others such as Night Calm in Egypt, is a medication used in the treatment of insomnia. Evidence supports slight to moderate benefit up to six months. It is taken orally.

Zaleplon, sold under the brand names Sonata among others, is a sedative-hypnotic, used to treat insomnia. It is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class.

Trazodone, sold under many brand names, is an antidepressant medication. It is used to treat major depressive disorder, anxiety disorders, and difficulties with sleep. The medication is taken orally.

Ramelteon, sold under the brand name Rozerem among others, is a melatonin agonist medication which is used in the treatment of insomnia. It is indicated specifically for the treatment of insomnia characterized by difficulties with sleep onset. It reduces the time taken to fall asleep, but the degree of clinical benefit is small. The medication is approved for long-term use. Ramelteon is taken by mouth.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones.

Bifeprunox (INN) (code name DU-127,090) is an atypical antipsychotic which, similarly to aripiprazole, combines minimal D2 receptor agonism with serotonin receptor agonism. It was under development for the treatment of schizophrenia but has since been abandoned.

Dianicline (SSR-591,813) is a drug developed by Sanofi-Aventis which acts as a partial agonist at neural nicotinic acetylcholine receptors. It is subtype-selective, binding primarily to the α4β2 subtype. It is being developed as a medication for the treatment of nicotine dependence to assist in smoking cessation. Dianicline is very similar to the already marketed drug varenicline and it is unclear what advantages it will have over the older drug, although it may have an improved side effect profile. It has been through human trials up to Phase II, although results have not yet been reported. Drug development has been discontinued after reporting of unfavourable results during Phase III trials.

Vilazodone, sold under the brand name Viibryd among others, is a medication used to treat major depressive disorder. It is taken by mouth.
Pitolisant, sold under the brand name Wakix among others, is a medication for the treatment of excessive daytime sleepiness in adults with narcolepsy. It is a histamine 3 (H3) receptor antagonist/inverse agonist. It represents the first commercially available medication in its class. Pitolisant enhances the activity of histaminergic neurons in the brain that function to improve a person's wakefulness.

Pimavanserin , sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis and is also being studied for the treatment of Alzheimer’s disease psychosis, schizophrenia, agitation, and major depressive disorder. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist.
Tiospirone (BMY-13,859), also sometimes called tiaspirone or tiosperone, is an atypical antipsychotic of the azapirone class. It was investigated as a treatment for schizophrenia in the late 1980s and was found to have an effectiveness equivalent to those of typical antipsychotics in clinical trials but without causing extrapyramidal side effects. However, development was halted and it was not marketed. Perospirone, another azapirone derivative with antipsychotic properties, was synthesized and assayed several years after tiospirone. It was found to be both more potent and more selective in comparison and was commercialized instead.

Esmirtazapine (ORG-50,081) is a drug which was under development by Organon for the treatment of insomnia and vasomotor symptoms (e.g., hot flashes) associated with menopause. Esmirtazapine is the (S)-(+)-enantiomer of mirtazapine and possesses similar overall pharmacology, including inverse agonist actions at H1 and 5-HT2 receptors and antagonist actions at α2-adrenergic receptors.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.

Nelotanserin is a drug developed by Arena Pharmaceuticals which acts as an inverse agonist on the serotonin receptor subtype 5-HT2A and was under development for the treatment of insomnia. It was shown to be effective and well tolerated in clinical trials, but development was halted in December 2008 because the substance did not meet the trial's effectiveness endpoints. Research continues on newer analogues which may potentially be more successful. More recently, nelotanserin has been repurposed for the treatment of Lewy body disease. As of 2017, it is in phase II clinical trials for this indication.
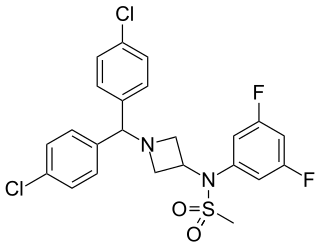
Drinabant (INN; AVE-1625) is a drug that acts as a selective CB1 receptor antagonist, which was under investigation varyingly by Sanofi-Aventis as a treatment for obesity, schizophrenia, Alzheimer's disease, Parkinson's disease, and nicotine dependence. Though initially studied as a potential treatment for a variety of different medical conditions, Sanofi-Aventis eventually narrowed down the therapeutic indications of the compound to just appetite suppression. Drinabant reached phase IIb clinical trials for this purpose in the treatment of obesity but was shortly thereafter discontinued, likely due to the observation of severe psychiatric side effects including anxiety, depression, and thoughts of suicide in patients treated with the now-withdrawn rimonabant, another CB1 antagonist that was also under development by Sanofi-Aventis.

TIK-301 (LY-156735) is an agonist for the melatonin receptors MT1 and MT2 that is under development for the treatment of insomnia and other sleep disorders. Its agonist action on MT1 and MT2 receptors in the suprachiasmatic nucleus in the brain enables its action as a chronobiotic. It is in the same class of melatonin receptor agonists as ramelteon and tasimelteon.
Piromelatine (Neu-P11) is a multimodal sleep drug under development by Neurim Pharmaceuticals. It is an agonist at melatonin MT1/MT2 and serotonin 5-HT1A/5-HT1D receptors. Neurim is conducting a phase II randomized, placebo controlled trial of cognitive and sleep effects in Alzheimer's disease.

Daridorexant, sold under the brand name Quviviq, is an orexin antagonist medication which is used for the treatment of insomnia. Daridorexant is taken by mouth.