
Kavalactones are a class of lactone compounds found in kava roots and Alpinia zerumbet (shell ginger). [1] Some kavalactones are bioactive. [2] [3]

Kavalactones are a class of lactone compounds found in kava roots and Alpinia zerumbet (shell ginger). [1] Some kavalactones are bioactive. [2] [3]
Kava extract interacts with many pharmaceuticals and herbal medications. In human volunteers, in vivo inhibition includes CYP1A2 [4] and CYP2E1 [5] through use of probe drugs to measure inhibition.
Its anxiolytic and hepatotoxicity activities have been investigated. [6] [7] [8]
The major kavalactones (except for desmethoxyyangonin) potentiate GABAA receptors, which may underlie the anxiolytic and sedative properties of kava. Further, inhibition of the reuptake of norepinephrine and dopamine, binding to the CB1 receptor, [9] inhibition of voltage-gated sodium and calcium channels, and monoamine oxidase B reversible inhibition are additional pharmacological actions that have been reported for kavalactones. [10]
Kavalactone-type compounds may help protect against high glucose induced cell damage. [2]
Several kavalactones (e.g., methysticin and yangonin) affect a group of enzymes involved in metabolism, called the CYP450 system. Hepatotoxicity occurred in a small portion of previously healthy kava users, [7] [11] particularly from extracts, as opposed to whole root powders.
At least 18 different kavalactones are known, [1] with methysticin being the first identified. [12] Multiple analogues, such as ethysticin, have also been isolated. [13] Some consist of a substituted α-pyrone as the lactone, while others are partially saturated.
The average elimination half-life of kavalactones typically present in kava root is 9 hr. [14]
| Name | Structure | R1 | R2 | R3 | R4 |
|---|---|---|---|---|---|
| Yangonin | 1 | -OCH3 | -H | -H | -H |
| 10-methoxyyangonin | 1 | -OCH3 | -H | -OCH3 | -H |
| 11-methoxyyangonin | 1 | -OCH3 | -OCH3 | -H | -H |
| 11-hydroxyyangonin | 1 | -OCH3 | -OH | -H | -H |
| Desmethoxyyangonin | 1 | -H | -H | -H | -H |
| 11-methoxy-12-hydroxydehydrokavain | 1 | -OH | -OCH3 | -H | -H |
| 7,8-dihydroyangonin | 2 | -OCH3 | -H | -H | -H |
| Kavain | 3 | -H | -H | -H | -H |
| 5-hydroxykavain | 3 | -H | -H | -H | -OH |
| 5,6-dihydroyangonin | 3 | -OCH3 | -H | -H | -H |
| 7,8-dihydrokavain | 4 | -H | -H | -H | -H |
| 5,6,7,8-tetrahydroyangonin | 4 | -OCH3 | -H | -H | -H |
| 5,6-dehydromethysticin | 5 | -O-CH2-O- | -H | -H | |
| Methysticin | 7 | -O-CH2-O- | -H | -H | |
| 7,8-dihydromethysticin | 8 | -O-CH2-O- | -H | -H | |
 |  |  |  |
 |  |  |  |
The kavalactone biosynthetic pathway in Piper methysticum was described in 2019. [15]

Piper, the pepper plants or pepper vines, is an economically and ecologically important genus in the family Piperaceae.

Kava or kava kava is a crop of the Pacific Islands. The name kava is from Tongan and Marquesan, meaning 'bitter'; other names for kava include ʻawa (Hawaiʻi), ʻava (Samoa), yaqona or yagona (Fiji), sakau (Pohnpei), seka (Kosrae), and malok or malogu. Kava is consumed for its sedating effects throughout the Pacific Ocean cultures of Polynesia, including Hawaii and Vanuatu, Melanesia, some parts of Micronesia, such as Pohnpei and Kosrae, and the Philippines.

Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.
Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by CYP3A4 gene. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.

Alpinia zerumbet, commonly known as shell ginger among other names, is a perennial species of ginger native to East Asia. The plants can grow up to 2.5 to 3 meters tall and bear colorful funnel-shaped flowers. They are grown as ornamentals and their leaves are used in cuisine and traditional medicine.
Cytochrome P450 1A2, a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the human body. In humans, the CYP1A2 enzyme is encoded by the CYP1A2 gene.

Cytochrome P450 2C19 is an enzyme protein. It is a member of the CYP2C subfamily of the cytochrome P450 mixed-function oxidase system. This subfamily includes enzymes that catalyze metabolism of xenobiotics, including some proton pump inhibitors and antiepileptic drugs. In humans, it is the CYP2C19 gene that encodes the CYP2C19 protein. CYP2C19 is a liver enzyme that acts on at least 10% of drugs in current clinical use, most notably the antiplatelet treatment clopidogrel (Plavix), drugs that treat pain associated with ulcers, such as omeprazole, antiseizure drugs such as mephenytoin, the antimalarial proguanil, and the anxiolytic diazepam.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice.

TPA-023 (MK-0777) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a mixed, subtype-selective ligand of the benzodiazepine site of α1, α2, α3, and α5-containing GABAA receptors, where it acts as a partial agonist at benzodiazepine sites of the α2 and α3-containing subtypes, but as a silent antagonist at α1 and α5-containing subtypes. It has primarily anxiolytic and anticonvulsant effects in animal tests, but with no sedative effects even at 50 times the effective anxiolytic dose.

Desmethoxyyangonin or 5,6-dehydrokavain is one of the six main kavalactones found in the Piper methysticum (kava) plant.

Kavain is the main kavalactone found mostly in the roots of the kava plant.

Baicalein (5,6,7-trihydroxyflavone) is a flavone, a type of flavonoid, originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora. It is also reported in Oroxylum indicum and Thyme. It is the aglycone of baicalin. Baicalein is one of the active ingredients of Sho-Saiko-To, which is a Chinese classic herbal formula, and listed in Japan as Kampo medicine.

Pipermethystine is a toxic alkaloid present in the aerial (aboveground) portions of the kava plant. It is not a kavalactone, containing no lactone structure. Correctly prepared kava root products will contain almost no pipermethystine.
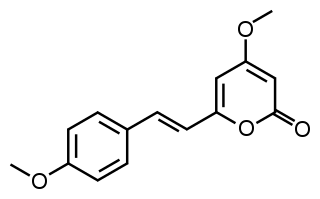
Yangonin is one of the six major kavalactones found in the kava plant. It has been shown to possess binding affinity for the cannabinoid receptor CB1 (Ki = 0.72 μM), and selectivity vs. the CB2 receptor (Ki >10 μM) where it behaves as an agonist. The CB1 receptor affinity of yangonin suggests that the endocannabinoid system might contribute to the complex human psychopharmacology of the traditional kava drink and the anxiolytic preparations obtained from the kava plant.

Dihydromethysticin is one of the six major kavalactones found in the kava plant.

Methysticin is one of the six major kavalactones found in the kava plant. Research suggests that methysticin and the related compound dihydromethysticin have CYP1A1 inducing effects which may be responsible for their toxicity. Additionally, methysticin has been shown to potentiate GABAA receptor activity, contributing to the overall anxiolytic profile of the kava plant.

Flavokavain A is a flavokavain found in the kava plant. It induces apoptosis in bladder cancer cells via a Bax protein-dependent and mitochondria-dependent apoptotic pathway.

Flavokavain B is a flavokavain found in the kava plant. In 2010 a paper was published identifying it as a glutathione-depleting hepatotoxin.
Jerome Sarris is co-director of Psychae Institute, Professor of Integrative Mental Health at Western Sydney University, Australia, and a visiting scientist at the Florey Institute of Neuroscience and Mental Health at the University of Melbourne, Australia.