Related Research Articles

Tannins are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids.

Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Ellagic acid is a polyphenol found in numerous fruits and vegetables. It is the dilactone of hexahydroxydiphenic acid.

Punicalagin (Pyuni-cala-jen) is an ellagitannin, a type of phenolic compound. It is found as alpha and beta isomers in pomegranates, Terminalia catappa, Terminalia myriocarpa, and in Combretum molle, the velvet bushwillow, a plant species found in South Africa. These three genera are all Myrtales and the last two are both Combretaceae.
The enzyme tannase (EC 3.1.1.20) catalyzes the following reaction:

Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.
A hydrolysable tannin or pyrogallol-type tannin is a type of tannin that, on heating with hydrochloric or sulfuric acids, yields gallic or ellagic acids.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.

Hexahydroxydiphenic acid is an organic compound with the formula [(HO)3C6HCO2H]2. It is the oxidatively coupled derivative of gallic acid It is a white solid, although samples are typically brown owing to oxidation.

1,2,3,4,6-Pentagalloylglucose is the pentagallic acid ester of glucose. It is a gallotannin and the precursor of ellagitannins.

Punigluconin is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate) and in Emblica officinalis. It is a molecule having a hexahydroxydiphenic acid group and two gallic acids attached to a gluconic acid core.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of the pomegranate fruit.

Pedunculagin is an ellagitannin. It is formed from casuarictin via the loss of a gallate group.
Maximilian Nierenstein was a professor of biochemistry at the University of Bristol.

Sanguisorbic acid is a constituent of some ellagitannins. It is constituted by a hexahydroxydiphenic acid unit linked by an O-C bond to a gallic acid. The differences with its isomers, valoneic acid and nonahydroxytriphenic acid, are that the hydroxyl that links the hexahydroxydiphenoyl (HHDP) group to the galloyl group belongs to the galloyl group in valoneic acid, while in nonahydroxytriphenic acid, the hexahydroxydiphenic acid unit is linked by a C-C bond to gallic acid.
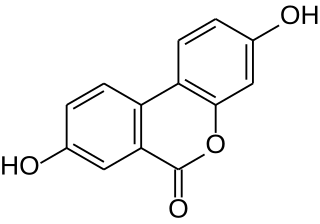
Urolithins are microflora metabolites of dietary ellagic acid derivatives, such as ellagitannins. They are produced in the gut, and found in the urine in the form of urolithin B glucuronide after absorption of ellagitannins-containing foods, such as pomegranate. During intestinal metabolism by bacteria, ellagitannins and punicalagins are converted to urolithins, which have unknown biological activity in vivo.

Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria. It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, walnuts, and others.

Tellimagrandin I is an ellagitannin found in plants, such as Cornus canadensis, Eucalyptus globulus, Melaleuca styphelioides, Rosa rugosa, and walnut. It is composed of two galloyl and one hexahydroxydiphenyl groups bound to a glucose residue. It differs from Tellimagrandin II only by a hydroxyl group instead of a third galloyl group. It is also structurally similar to punigluconin and pedunculagin, two more ellagitannin monomers.
References
- ↑ MALDI-TOF Mass Spectrometric Analysis of Hydrolysable Tannins
- ↑ Structural diversity and antimicrobial activities of ellagitannins. T. Yoshida, Ts. Hatano, H. Ito, T. Okuda, S. Quideau (Ed.), Chemistry and Biology of Ellagitannins, World Scientific Publishing, Singapore (2009), pages 55–93
- ↑ Davis, CD; Milner, JA (Oct 2009). "Gastrointestinal microflora, food components and colon cancer prevention". J Nutr Biochem. 20 (10): 743–52. doi:10.1016/j.jnutbio.2009.06.001. PMC 2743755 . PMID 19716282.
- ↑ Yoshida, Takashi (2010). "Structural Features and Biological Properties of Ellagitannins in Some Plant Families of the Order Myrtales". International Journal of Molecular Sciences. 11 (1): 79–106. doi: 10.3390/ijms11010079 . PMC 2820991 . PMID 20162003.
- ↑ Gómez-Caravaca, A. M.; Verardo, V; Toselli, M; Segura-Carretero, A; Fernández-Gutiérrez, A; Caboni, M. F. (2013). "Determination of the major phenolic compounds in pomegranate juices by HPLC−DAD−ESI-MS". Journal of Agricultural and Food Chemistry. 61 (22): 5328–37. doi:10.1021/jf400684n. PMID 23656584.