Polyphenols are a structural class of mainly natural, but also synthetic or semisynthetic, organic chemicals characterized by the presence of large multiples of phenol structural units. The number and characteristics of these phenol structures underlie the unique physical, chemical, and biological properties of particular members of the class. Examples include tannic acid and ellagitannin. The historically important chemical class of tannins is a subset of the polyphenols.

Chlorogenic acid (CGA) is the ester of caffeic acid and (−)-quinic acid, functioning as an intermediate in lignin biosynthesis. The term "chlorogenic acids" refers to a related polyphenol family of esters, including hydroxycinnamic acids with quinic acid.

Ellagic acid is a natural phenol antioxidant found in numerous fruits and vegetables. The antiproliferative and antioxidant properties of ellagic acid have prompted research into its potential health benefits. Ellagic acid is the dilactone of hexahydroxydiphenic acid.

Punicalagin is an ellagitannin, a type of phenolic compound. It is found in forms alpha and beta in pomegranates, in Terminalia catappa and Terminalia myriocarpa, and in Combretum molle, the velvet bushwillow, a plant species found in South Africa. These three genera are all Myrtales and the last two are both Combretaceae.
Proanthocyanidins are a class of polyphenols found in a variety of plants. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.
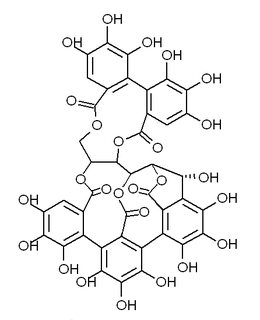
Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.
The ellagitannins are a diverse class of hydrolyzable tannins, a type of polyphenol formed primarily from the oxidative linkage of galloyl groups in 1,2,3,4,6-pentagalloyl glucose. Ellagitannins differ from gallotannins, in that their galloyl groups are linked through C-C bonds, whereas the galloyl groups in gallotannins are linked by depside bonds.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.
Tergallic acids are trimers of gallic acid, often found naturally in the form of glycosides. Tergallic acid O- or C-glucosides that can be found in acorns of several Quercus (oak) species. The dehydrated tergallic acid C-glucoside and tergallic acid O-glucoside can be characterised in the acorns of Quercus macrocarpa. Dehydrated tergallic-C-glucoside can be found in the cork from Quercus suber.

δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine leaves. It is also found in plant cell cultures. or in wine. It can also be found in Rheum maximowiczii.

Punicalin is an ellagitannin. It can be found in Punica granatum (pomegranate) or in the leaves of Terminalia catappa, a plant used to treat dermatitis and hepatitis. It is also reported in Combretum glutinosum, all three species being Myrtales, the two last being Combretaceae.

Pedunculagin is an ellagitannin. It is formed from casuarictin via the loss of a gallate group.

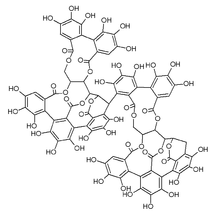
Lambertianin C is an ellagitannin.

Sanguiin H-6 is an ellagitannin.

Urolithins are microflora human metabolites of dietary ellagic acid derivatives such as ellagitannins. They are produced in the human gut, and found in the urine in the form of urolithin B glucuronide after absorption of ellagitannins-containing food such as pomegranate, strawberries, red raspberries, walnuts or oak-aged red wine.

Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria. It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, and walnuts. Since the 2000s, urolithin A has been subject of preliminary studies regarding its possible biological effects.