
Tara spinosa, commonly known as tara (Quechua), also known as Peruvian carob or spiny holdback, is a small leguminous tree or thorny shrub native to Peru. T. spinosa is cultivated as a source of tannins based on a galloylated quinic acid structure. This chemical structure has been confirmed also by LC-MS. It is also grown as an ornamental plant because of its large colorful flowers and pods.
The enzyme tannase (EC 3.1.1.20) catalyzes the following reaction:

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Chebulinic acid is an ellagitannin found in the seeds of Euphoria longana, in the fruits of Terminalia chebula or in the leaves of T. macroptera.
A gallotannin is any of a class of molecules belonging to the hydrolysable tannins. Gallotannins are polymers formed when gallic acid, a polyphenol monomer, esterifies and binds with the hydroxyl group of a polyol carbohydrate such as glucose.
A hydrolyzable tannin or pyrogallol-type tannin is a type of tannin that, on heating with hydrochloric or sulfuric acids, yields gallic or ellagic acids.
The ellagitannins are a diverse class of hydrolyzable tannins, a type of polyphenol formed primarily from the oxidative linkage of galloyl groups in 1,2,3,4,6-pentagalloyl glucose. Ellagitannins differ from gallotannins, in that their galloyl groups are linked through C-C bonds, whereas the galloyl groups in gallotannins are linked by depside bonds.

Casuarictin is an ellagitannin, a type of hydrolysable tannin. It can be found in Casuarina and Stachyurus species.
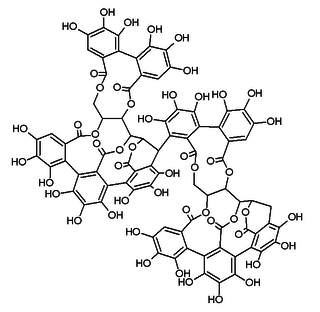
Roburin A is a tannin found in oak wood or oak cork.
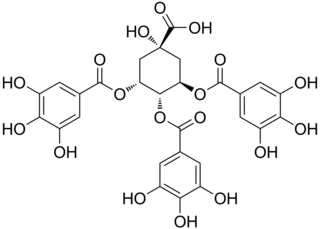
3,4,5-Tri-O-galloylquinic acid is a hydrolysable tannin found in Lepidobotrys staudtii, in Guiera senegalensis or in the resurrection plant.

Terflavin B is an ellagitannin, a type of hydrolysable tannin. It can be found in Myrobalanus chebula, the black chebulic, and in Terminalia catappa, the Indian almond.
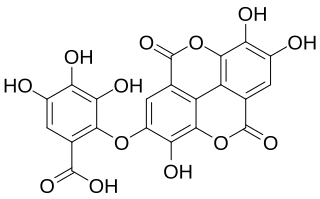
Valoneic acid dilactone is a hydrolysable tannin that can be isolated from the heartwood of Shorea laeviforia and in oaks species like the North American white oak and European red oak.
Shorea laeviforia is a plant species in the genus Shorea.

Flavogallonic acid is a hydrolysable tannin that can be found in valonea oak in chestnut wood or in Terminalia myriocarpa.
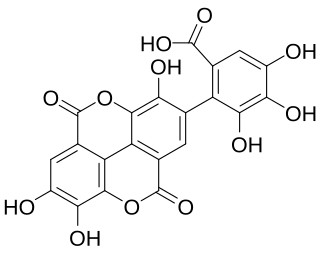
Flavogallonic acid dilactone is a hydrolysable tannin that can be found in Rhynchosia volubilis seeds, in Shorea laeviforia, in Anogeissus leiocarpus and Terminalia avicennoides.

Bicornin is an ellagitannin found in the Myrtales Trapa bicornis and Syzygium aromaticum (clove).
The molecular formula C21H14O15 (molar mass: 506.33 g/mol, exact mass: 506.03327 u) may refer to:
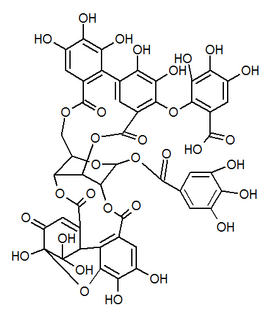
Mallotusinic acid is a hydrolysable tannin found in the bark of Mallotus japonicus. It is more generally present in Geraniales.
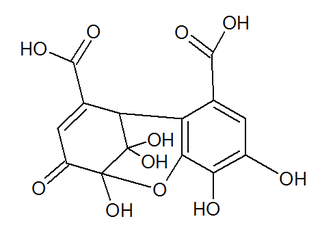
Dehydrohexahydroxydiphenic acid is a group found in dehydroellagitannins. It is formed from hexahydroxydiphenic acid (HHDP) through oxidation of the plant hydrolysable tannins. It is found in ellagitannins such as euphorbin A, geraniin or mallotusinic acid.

Mallojaponin is a hydrolysable tannin found in the bark of Mallotus japonicus. This compound contains the moiety elaeocarpusinic acid, an oxidized hexahydroxydiphenic acid group which reacted with a dehydroascorbic acid molecule. It also contains a valoneic acid and a gallic acid moieties linked to a glucose molecule.















