Ellagic acid is a polyphenol found in numerous fruits and vegetables. It is the dilactone of hexahydroxydiphenic acid.

Carbonic anhydrase inhibitors are a class of pharmaceuticals that suppress the activity of carbonic anhydrase. Their clinical use has been established as anti-glaucoma agents, diuretics, antiepileptics, in the management of mountain sickness, gastric and duodenal ulcers, idiopathic intracranial hypertension, neurological disorders, or osteoporosis.
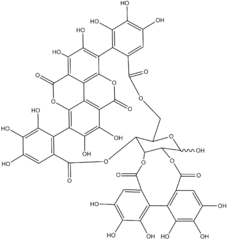
1-α-O-Galloylpunicalagin is an ester of gallic acid and punicalagin, a type of ellagitannin. It is found in the pomegranate and in Combretum glutinosum.
A hydrolysable tannin or pyrogallol-type tannin is a type of tannin that, on heating with hydrochloric or sulfuric acids, yields gallic or ellagic acids.
The ellagitannins are a diverse class of hydrolyzable tannins, a type of polyphenol formed primarily from the oxidative linkage of galloyl groups in 1,2,3,4,6-pentagalloyl glucose. Ellagitannins differ from gallotannins, in that their galloyl groups are linked through C-C bonds, whereas the galloyl groups in gallotannins are linked by depside bonds.

Corilagin is an ellagitannin. Corilagin was first isolated in 1951 from Dividivi extract and from Caesalpinia coriaria, hence the name of the molecule. It can also be found in Alchornea glandulosa and in the leaves of Punica granatum (pomegranate).

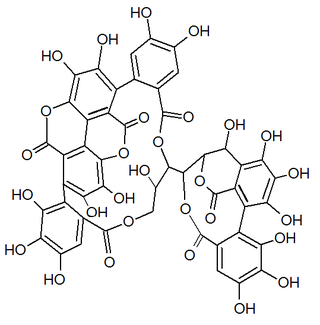
Gallagic acid is a polyphenolic chemical compound that can be found in the ellagitannins, a type of tannin, found in Punica granatum (pomegranate). It is a building block of the corresponding tannin punicalagin, punicalin, punicacortein C and 2-O-galloyl-punicalin.

Punigluconin is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate) and in Emblica officinalis. It is a molecule having a hexahydroxydiphenic acid group and two gallic acids attached to a gluconic acid core.

Punicalin is an ellagitannin. It can be found in Punica granatum (pomegranate) or in the leaves of Terminalia catappa, a plant used to treat dermatitis and hepatitis. It is also reported in Combretum glutinosum, all three species being Myrtales, the two last being Combretaceae.
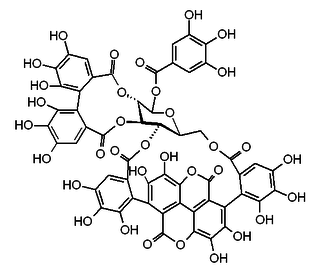
Punicacortein C is an ellagitannin, a phenolic compound. It is found in the bark of Punica granatum (pomegranate). The molecule contains a gallagic acid component.

Punicacortein D is an ellagitannin, a type of phenolic compound. It is found in the bark and heartwood of Punica granatum (pomegranate). The molecule contains a gallagic acid component.

Granatin A is an ellagitannin found in the pericarp of Punica granatum (pomegranate). It is a weak carbonic anhydrase inhibitor.

Granatin B is an ellagitannin found in the fruit of Punica granatum (pomegranate). It is a molecule having an enantiomeric dehydrohexahydroxydiphenoyl group.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of pomegranates.

Combretum molle, the velvet bushwillow, is a medium to large tree species in the genus Combretum found in western, eastern and southern Africa.

Terminalia myriocarpa, the East Indian almond, is a tree species in the genus Terminalia found in Southeast Asia.

Casuarinin is an ellagitannin. It is found in the pericarp of pomegranates. It is also found in Casuarina and Stachyurus species and in Alnus sieboldiana.

Pedunculagin is an ellagitannin. It is formed from casuarictin via the loss of a gallate group.

Methyl gallate is a phenolic compound. It is the methyl ester of gallic acid.
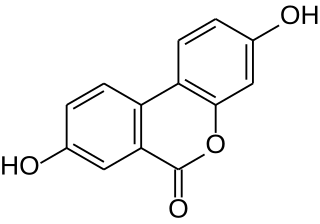
Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria. It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, walnuts, and others.