
Beavers are large, semiaquatic rodents in the genus Castor native to the temperate Northern Hemisphere. There are two extant species: the North American beaver and the Eurasian beaver. Beavers are the second-largest living rodents after the capybaras. They have stout bodies with large heads, long chisel-like incisors, brown or gray fur, hand-like front feet, webbed back feet and flat, scaly tails. The Eurasian beaver has a more elongated skull with a more triangular nasal bone opening, lighter fur color and a narrower tail. The animals can be found in a number of freshwater habitats, such as rivers, streams, lakes and ponds. They are herbivorous, consuming tree bark, aquatic plants, grasses and sedges.

Ricinus communis, the castor bean or castor oil plant, is a species of perennial flowering plant in the spurge family, Euphorbiaceae. It is the sole species in the monotypic genus, Ricinus, and subtribe, Ricininae. The evolution of castor and its relation to other species are currently being studied using modern genetic tools. It reproduces with a mixed pollination system which favors selfing by geitonogamy but at the same time can be an out-crosser by anemophily or entomophily.

Castor oil is a vegetable oil pressed from castor beans. Castor oil is a colourless to very pale yellow liquid with a distinct taste and odor. Its boiling point is 313 °C (595 °F) and its density is 0.961 g/cm3. It includes a mixture of triglycerides in which approximately 90 percent of fatty acid chains are ricinoleates. Oleate and linoleates are the other significant components.

The Eurasian beaver or European beaver is a beaver species that was once widespread in Eurasia, but was hunted to near-extinction for both its fur and castoreum. At the turn of the 20th century, only about 1,200 beavers survived in eight relict populations in Europe and Asia. It has been reintroduced to much of its former range, and now occurs from Spain, Central Europe, Great Britain and Scandinavia to a few regions in China and Mongolia. It is listed as least concern on the IUCN Red List, as it recovered well in most of Europe. It is extinct in Portugal, Moldova, and Turkey.

Parabens are a class of widely used preservatives in cosmetic and pharmaceutical products. Chemically, they are a series of parahydroxybenzoates or esters of parahydroxybenzoic acid. Parabens are effective preservatives in many types of formulas. These compounds, and their salts, are used primarily for their bactericidal and fungicidal properties. They are found in shampoos, commercial moisturizers, shaving gels, personal lubricants, topical/parenteral pharmaceuticals, suntan products, makeup, and toothpaste. They are also used as food preservatives.

The North American beaver is one of two extant beaver species, along with the Eurasian beaver. It is native to North America and introduced in South America (Patagonia) and Europe. In the United States and Canada, the species is often referred to simply as "beaver", though this causes some confusion because another distantly related rodent, Aplodontia rufa, is often called the "mountain beaver". Other vernacular names, including American beaver and Canadian beaver, distinguish this species from the other extant beaver species, Castor fiber, which is native to Eurasia. The North American beaver is one of the official wildlife of Canada symbols and is the official state mammal of Oregon and New York.

Castoreum is a yellowish exudate from the castor sacs of mature beavers. Beavers use castoreum in combination with urine to scent mark their territory. Both beaver sexes have a pair of castor sacs and a pair of anal glands, located in two cavities under the skin between the pelvis and the base of the tail. The castor sacs are not true glands on a cellular level, hence references to these structures as preputial glands, castor glands, or scent glands are misnomers.
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a key precursor to a variety chelating agents, some of which are commercially important.
4-Ethylphenol (4-EP) is a phenolic compound.
Monohydroxybenzoic acid may refer to any of three isomeric phenolic acids:

Fenamic acid is an organic compound, which, especially in its ester form, is called fenamate. serves as a parent structure for several nonsteroidal anti-inflammatory drugs (NSAIDs), including mefenamic acid, tolfenamic acid, flufenamic acid, and meclofenamic acid. These drugs are commonly referred to as "anthranilic acid derivatives" or "fenamates" because fenamic acid is a derivative of anthranilic acid.

Phenolic acids or phenolcarboxylic acids are types of aromatic acid compound. Included in that class are substances containing a phenolic ring and an organic carboxylic acid function. Two important naturally occurring types of phenolic acids are hydroxybenzoic acids and hydroxycinnamic acids, which are derived from non-phenolic molecules of benzoic and cinnamic acid, respectively.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
Nonadecylic acid, or nonadecanoic acid, is a 19-carbon saturated fatty acid with the chemical formula CH3(CH2)17COOH. It forms salts called nonadecylates. Nonadecylic acid can be found in fats and vegetable oils, although it is rare.
ortho-Cresol, also 2-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and m-cresol.

5-Methoxysalicylic acid is a chemical compound. It is a component of castoreum, the exudate from the castor sacs of the mature beaver.
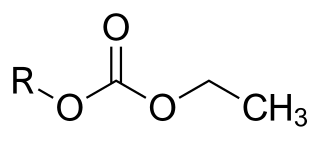
Etabonate or ethyl carbonate is the chemical group with formula –CO
3–C
2H
5, or H
3C–CH
2–O–C(=O)–O–. The names are also used for esters R–OCO
2C
2H
5, for the anion [C
2H
5OCO−
2], and for salts of the latter.

Agnuside is a chemical compound found in Vitex agnus-castus. Agnuside is the ester of aucubin and p-hydroxybenzoic acid.

4-Hydroxybenzoic acid 4-O-glucoside is a glucoside of p-hydroxybenzoic acid. It can be found in mycorrhizal and non-mycorrhizal roots of Norway spruces.













