The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR)., and independently by Heinrich Hock in 1944
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

An epoxide is a cyclic ether with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone, however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

A sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

A peroxy acid is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy derivatives of organic carboxylic acids. They are generally strong oxidizers.

Anthrone is a tricyclic aromatic ketone. It is used for a common cellulose assay and in the colorometric determination of carbohydrates.
Peracetic acid (also known as peroxyacetic acid, or PAA), is an organic compound with the formula CH3CO3H. This organic peroxide is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive.
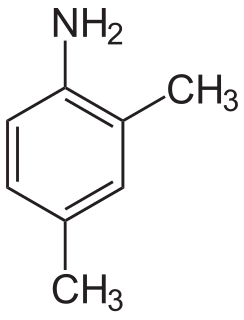
2,4-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives include the veterinary drug cymiazole and the colorant Pigment Yellow 81.

Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially.
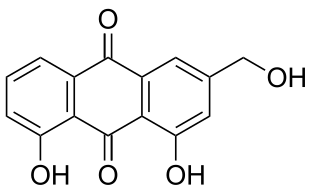
Aloe emodin is an anthraquinone and an isomer of emodin present in aloe latex, an exudate from the aloe plant. It has a strong stimulant-laxative action. Aloe emodin is not carcinogenic when applied to the skin, although it may increase the carcinogenicity of some kind of radiation.

2-Ethylanthraquinone is an organic compound that is a derivative of anthraquinone. This pale yellow solid is used in the industrial production of hydrogen peroxide (H2O2).

Pyrethrin II is an organic compound that is a potent insecticide. It is one of the two pyrethrins, the other being pyrethrin I. Thousands of tons this mixture are produced annually from chrysanthemum plants, which are cultivated in warm climates. Whereas pyrethrin I is a derivative of (+)-trans-chrysanthemic acid, in pyrethrin II one methyl group is oxidized to a carboxymethyl group, the resulting core being called pyrethric acid. Knowledge of their chemical structures opened the way for the production of synthetic analogues, which are called pyrethroids. In terms of their biosynthesis, pyrethrins are classified as terpenoids, being derived from dimethylallyl pyrophosphate.
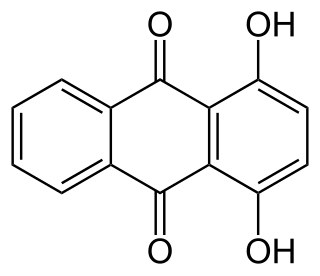
1,4-Dihydroxyanthraquinone, also called quinizarin or Solvent Orange 86, is an organic compound derived from anthroquinone. Quinizarin is an orange or red-brown crystalline powder. It is formally derived from anthraquinone by replacement of two hydrogen atoms by hydroxyl (OH) groups. It is one of ten dihydroxyanthraquinone isomers and occurs in small amounts in the root of the madder plant, Rubia tinctorum.

A hydroxyanthraquinone (formula: C14H9O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).

9,10-Dihydroxyanthracene is an organic compound with the formula (C6H4CHOH)2. It is the hydroquinone form of 9,10-anthraquinone (AQ). It formed when AQ is hydrogenated. It is easily dissolved in alkaline solutions and is often called soluble anthraquinone (SAQ).

The anthraquinone process is a process for the production of hydrogen peroxide, which was developed by BASF. The industrial production of hydrogen peroxide is based on the reduction of oxygen, as in the direct synthesis from the elements. Instead of hydrogen itself, however, a 2-alkyl-anthrahydroquinone, which is generated before from the corresponding 2-alkyl-anthraquinone by catalytic hydrogenation with palladium is used. Oxygen and the organic phase react under formation of the anthraquinone and hydrogen peroxide. Among other alkyl groups (R) ethyl- and tert-butyl- are used, e.g., 2-ethylanthraquinone.

Hydroxyanthracenes are a class of natural phenolic compounds. They can be found in Cassia alata and Cassia senna.
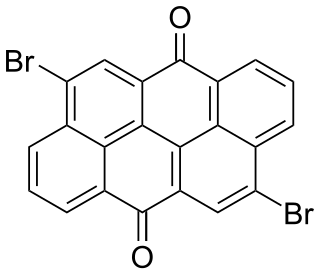
Dibromoanthanthrone is a scarlet or orange-red-hue synthetic organic colourant.
4-Chlorophenol is an organic compound with the formula ClC6H4OH. It is one of three monochlorophenol isomers. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Its pKa is 9.14.