
L-Tyrosine or tyrosine or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA.

Alkaptonuria is a rare inherited genetic disease which is caused by a mutation in the HGD gene for the enzyme homogentisate 1,2-dioxygenase ; if a person inherits an abnormal copy from both parents, the body accumulates an intermediate substance called homogentisic acid in the blood and tissues. Homogentisic acid and its oxidized form alkapton are excreted in the urine, giving it an unusually dark color. The accumulating homogentisic acid causes damage to cartilage and heart valves, as well as precipitating as kidney stones and stones in other organs. Symptoms usually develop in people over 30 years old, although the dark discoloration of the urine is present from birth.

Cysteine dioxygenase (CDO) is a non-heme iron enzyme that catalyzes the conversion of L-cysteine to cysteine sulfinic acid. CDO plays an important role in cysteine catabolism, regulating intracellular levels of cysteine and responding changes in cysteine availability. As such, CDO is highly regulated and undergoes large changes in concentration and efficiency. It oxidizes cysteine to the corresponding sulfinic acid by activation of dioxygen, although the exact mechanism of the reaction is still unclear. In addition to being found in mammals, CDO also exists in some yeast and bacteria, although the exact function is still unknown. CDO has been implicated in various neurodegenerative diseases and cancers, which is likely related to cysteine toxicity.

Arbutus unedo is an evergreen shrub or small tree in the family Ericaceae, native to the Mediterranean Basin and Western Europe. The tree is well known for its fruits, the arbutus berry, which bear some resemblance to the strawberry, hence the common name strawberry tree. However, it is not closely related to true strawberries of the genus Fragaria.

Tyrosinemia or tyrosinaemia is an error of metabolism, usually inborn, in which the body cannot effectively break down the amino acid tyrosine. Symptoms of untreated tyrosinemia include liver and kidney disturbances. Without treatment, tyrosinemia leads to liver failure. Today, tyrosinemia is increasingly detected on newborn screening tests before any symptoms appear. With early and lifelong management involving a low-protein diet, special protein formula, and sometimes medication, people with tyrosinemia develop normally, are healthy, and live normal lives.

Ochronosis is a syndrome caused by the accumulation of homogentisic acid in connective tissues. The condition was named after the yellowish (ocher-like) discoloration of the tissue seen on microscopic examination. Macroscopically, though, the affected tissues appear bluish-grey because of a light-scattering phenomenon known as the Tyndall effect. The condition is most often associated with alkaptonuria, but can occur from exogenous administration of phenol complexes such as hydroquinone. It was first described by Rudolf Virchow in 1865.

4-Hydroxyphenylpyruvate dioxygenase (HPPD), also known as α-ketoisocaproate dioxygenase, is an Fe(II)-containing non-heme oxygenase that catalyzes the second reaction in the catabolism of tyrosine - the conversion of 4-hydroxyphenylpyruvate into homogentisate. HPPD also catalyzes the conversion of phenylpyruvate to 2-hydroxyphenylacetate and the conversion of α-ketoisocaproate to β-hydroxy β-methylbutyrate. HPPD is an enzyme that is found in nearly all aerobic forms of life.

Hawkinsinuria is an autosomal dominant metabolic disorder affecting the metabolism of tyrosine.
Phytotoxins are substances that are poisonous or toxic to the growth of plants. Phytotoxic substances may result from human activity, as with herbicides, or they may be produced by plants, by microorganisms, or by naturally occurring chemical reactions.

Nitisinone, sold under the brand name Orfadin among others, is a medication used to slow the effects of hereditary tyrosinemia type 1 (HT-1).

Xanthomonas campestris is a gram-negative, obligate aerobic bacterium that is a member of the Xanthomonas genus, which is a group of bacteria that are commonly known for their association with plant disease. The species is considered to be dominant amongst its genus, as it originally had over 140 identified pathovars and has been found to infect both monocotyledonous and dicotyledonous plants of economical value with various plant diseases. This includes "black rot" in cruciferous vegetables, bacterial wilt of turfgrass, bacterial blight, and leaf spot, for example.

Homogentisate 1,2-dioxygenase (homogentisic acid oxidase, homogentisate oxidase, homogentisicase) is an enzyme which catalyzes the conversion of homogentisate to 4-maleylacetoacetate. Homogentisate 1,2-dioxygenase or HGD is involved in the catabolism of aromatic rings, more specifically in the breakdown of the amino acids tyrosine and phenylalanine. HGD appears in the metabolic pathway of tyrosine and phenylalanine degradation once the molecule homogentisate is produced. Homogentisate reacts with HGD to produce maleylacetoacetate, which then is further used in the metabolic pathway. HGD requires the use of Fe2+ and O2 in order to cleave the aromatic ring of homogentisate.

4-Hydroxyphenylpyruvic acid (4-HPPA) is an intermediate in the metabolism of the amino acid phenylalanine. The aromatic side chain of phenylalanine is hydroxylated by the enzyme phenylalanine hydroxylase to form tyrosine. The conversion from tyrosine to 4-HPPA is in turn catalyzed by tyrosine aminotransferase. Additionally, 4-HPPA can be converted to homogentisic acid which is one of the precursors to ochronotic pigment.

Fumarylacetoacetic acid (fumarylacetoacetate) is an intermediate in the metabolism of tyrosine. It is formed through the conversion of maleylacetoacetate into fumarylacetoacetate by the enzyme maleylacetoacetate isomerase.

In enzymology, maleylacetoacetate isomerase is an enzyme that catalyzes the chemical reaction

Dioxygenases are oxidoreductase enzymes. Aerobic life, from simple single-celled bacteria species to complex eukaryotic organisms, has evolved to depend on the oxidizing power of dioxygen in various metabolic pathways. From energetic adenosine triphosphate (ATP) generation to xenobiotic degradation, the use of dioxygen as a biological oxidant is widespread and varied in the exact mechanism of its use. Enzymes employ many different schemes to use dioxygen, and this largely depends on the substrate and reaction at hand.

Tyrosinemia type III is a rare disorder caused by a deficiency of the enzyme 4-hydroxyphenylpyruvate dioxygenase, encoded by the gene HPD. This enzyme is abundant in the liver, and smaller amounts are found in the kidneys. It is one of a series of enzymes needed to break down tyrosine. Specifically, 4-hydroxyphenylpyruvate dioxygenase converts a tyrosine byproduct called 4-hydroxyphenylpyruvate to homogentisic acid. Characteristic features of type III tyrosinemia include mild mental retardation, seizures, and periodic loss of balance and coordination. Type III tyrosinemia is very rare; only a few cases have been reported.
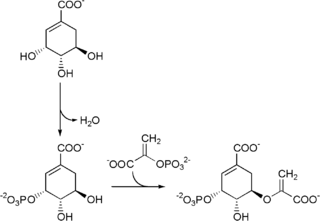
The shikimate pathway is a seven-step metabolic pathway used by bacteria, archaea, fungi, algae, some protozoans, and plants for the biosynthesis of folates and aromatic amino acids. This pathway is not found in animal cells.
4-Hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors are a class of herbicides that prevent growth in plants by blocking 4-Hydroxyphenylpyruvate dioxygenase, an enzyme in plants that breaks down the amino acid tyrosine into molecules that are then used by plants to create other molecules that plants need. This process of breakdown, or catabolism, and making new molecules from the results, or biosynthesis, is something all living things do. HPPD inhibitors were first brought to market in 1980, although their mechanism of action was not understood until the late 1990s. They were originally used primarily in Japan in rice production, but since the late 1990s have been used in Europe and North America for corn, soybeans, and cereals, and since the 2000s have become more important as weeds have become resistant to glyphosate and other herbicides. Genetically modified crops are under development that include resistance to HPPD inhibitors. There is a pharmaceutical drug on the market, nitisinone, that was originally under development as an herbicide as a member of this class, and is used to treat an orphan disease, type I tyrosinemia.

Tyrosinemia type I is a genetic disorder that disrupts the metabolism of the amino acid tyrosine, resulting in damage primarily to the liver along with the kidneys and peripheral nerves. The inability of cells to process tyrosine can lead to chronic liver damage ending in liver failure, as well as renal disease and rickets. Symptoms such as poor growth and enlarged liver are associated with the clinical presentation of the disease. If not detected via newborn screening and management not begun before symptoms appear, clinical manifestation of disease occurs typically within the first two years of life. The severity of the disease is correlated with the timing of onset of symptoms, earlier being more severe. If diagnosed through newborn screening prior to clinical manifestation, and well managed with diet and medication, normal growth and development is possible.


















