
Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent or it may aim to prolong life or to reduce symptoms. Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called medical oncology.
DNA topoisomerases are enzymes that catalyze changes in the topological state of DNA, interconverting relaxed and supercoiled forms, linked (catenated) and unlinked species, and knotted and unknotted DNA. Topological issues in DNA arise due to the intertwined nature of its double-helical structure, which, for example, can lead to overwinding of the DNA duplex during DNA replication and transcription. If left unchanged, this torsion would eventually stop the DNA or RNA polymerases involved in these processes from continuing along the DNA helix. A second topological challenge results from the linking or tangling of DNA during replication. Left unresolved, links between replicated DNA will impede cell division. The DNA topoisomerases prevent and correct these types of topological problems. They do this by binding to DNA and cutting the sugar-phosphate backbone of either one or both of the DNA strands. This transient break allows the DNA to be untangled or unwound, and, at the end of these processes, the DNA backbone is resealed. Since the overall chemical composition and connectivity of the DNA do not change, the DNA substrate and product are chemical isomers, differing only in their topology.

Idarubicin or 4-demethoxydaunorubicin is an anthracycline antileukemic drug. It inserts itself into DNA and prevents DNA unwinding by interfering with the enzyme topoisomerase II. It is an analog of daunorubicin, but the absence of a methoxy group increases its fat solubility and cellular uptake. Similar to other anthracyclines, it also induces histone eviction from chromatin.
Fluorouracil, sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts.

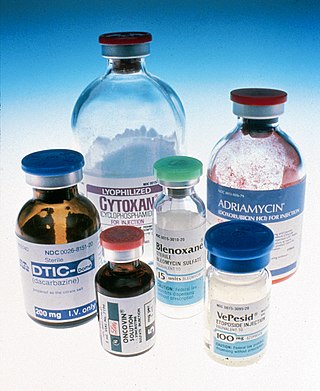
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.

An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid; thus, competitive inhibition can occur, and the presence of antimetabolites can have toxic effects on cells, such as halting cell growth and cell division, so these compounds are used in chemotherapy for cancer.
The era of cancer chemotherapy began in the 1940s with the first use of nitrogen mustards and folic acid antagonist drugs. The targeted therapy revolution has arrived, but many of the principles and limitations of chemotherapy discovered by the early researchers still apply.

Daunorubicin, also known as daunomycin, is a chemotherapy medication used to treat cancer. Specifically it is used for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), and Kaposi's sarcoma. It is administered by injection into a vein. A liposomal formulation known as liposomal daunorubicin also exists.

Epirubicin is an anthracycline drug used for chemotherapy. It can be used in combination with other medications to treat breast cancer in patients who have had surgery to remove the tumor. It is marketed by Pfizer under the trade name Ellence in the US and Pharmorubicin or Epirubicin Ebewe elsewhere.
Extravasation is the leakage of intravenously (IV) infused, and potentially damaging, medications into the extravascular tissue around the site of infusion. The leakage can occur through brittle veins in the elderly, through previous venipuncture access, or through direct leakage from wrongly positioned venous access devices. When the leakage is not of harmful consequence it is known as infiltration. Extravasation of medication during intravenous therapy is an adverse event related to therapy that, depending on the medication, amount of exposure, and location, can potentially cause serious injury and permanent harm, such as tissue necrosis. Milder consequences of extravasation include irritation, characterized by symptoms of pain and inflammation, with the clinical signs of warmth, erythema, or tenderness.
Cardiotoxicity is the occurrence of heart dysfunction as electric or muscle damage, resulting in heart toxicity. The heart becomes weaker and is not as efficient in pumping blood. Cardiotoxicity may be caused by chemotherapy treatment and/or radiotherapy; complications from anorexia nervosa; adverse effects of heavy metals intake; the long-term abuse of or ingestion at high doses of certain strong stimulants such as cocaine; or an incorrectly administered drug such as bupivacaine.

Dexrazoxane hydrochloride is a cardioprotective agent. It was discovered by Eugene Herman in 1972. The IV administration of dexrazoxane is in acidic condition with HCl adjusting the pH.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.

Breast cancer chemotherapy refers to the use of cytotoxic drugs (chemotherapy) in the treatment of breast cancer.
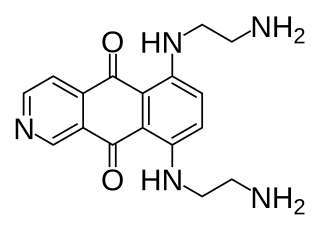
Pixantrone is an experimental antineoplastic (anti-cancer) drug, an analogue of mitoxantrone with fewer toxic effects on cardiac tissue. It acts as a topoisomerase II poison and intercalating agent. The code name BBR 2778 refers to pixantrone dimaleate, the actual substance commonly used in clinical trials.

TopoTarget was a Copenhagen-based biotechnology company focused on the discovery and development of drugs and therapies to treat cancer. In 2014, it merged with BioAlliance Pharma and is now part of Onxeo.

Follicular dendritic cell sarcoma (FDCS) is an extremely rare neoplasm. While the existence of FDC tumors was predicted by Lennert in 1978, the tumor wasn't fully recognized as its own cancer until 1986 after characterization by Monda et al. It accounts for only 0.4% of soft tissue sarcomas, but has significant recurrent and metastatic potential and is considered an intermediate grade malignancy. The major hurdle in treating FDCS has been misdiagnosis. It is a newly characterized cancer, and because of its similarities in presentation and markers to lymphoma, both Hodgkin and Non-Hodgkin subtypes, diagnosis of FDCS can be difficult. With recent advancements in cancer biology better diagnostic assays and chemotherapeutic agents have been made to more accurately diagnose and treat FDCS.

Daunorubicin/cytarabine is a fixed-dose combination medication used for the treatment of acute myeloid leukemia. It contains the liposomal bound daunorubicin, an anthracycline topoisomerase inhibitor, and cytarabine, a nucleoside metabolic inhibitor.
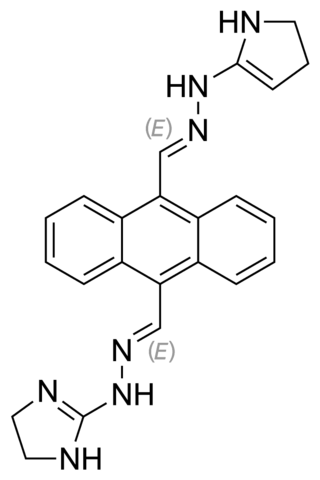
Bisantrene is an anthracenyl bishydrazone with anthracycline-like antineoplastic activity and an antimetabolite. Bisantrene intercalates with and disrupts the configuration of DNA, resulting in DNA single-strand breaks, DNA-protein crosslinking, and inhibition of DNA replication. This agent is similar to doxorubicin in chemotherapeutic activity, but unlike anthracyclines like doxorubicin, it exhibits little cardiotoxicity.

A ligand-targeted liposome (LTL) is a nanocarrier with specific ligands attached to its surface to enhance localization for targeted drug delivery. The targeting ability of LTLs enhances cellular localization and uptake of these liposomes for therapeutic or diagnostic purposes. LTLs have the potential to enhance drug delivery by decreasing peripheral systemic toxicity, increasing in vivo drug stability, enhancing cellular uptake, and increasing efficiency for chemotherapeutics and other applications. Liposomes are beneficial in therapeutic manufacturing because of low batch-to-batch variability, easy synthesis, favorable scalability, and strong biocompatibility. Ligand-targeting technology enhances liposomes by adding targeting properties for directed drug delivery.





















