Medicine
Biomarkers used in the medical field, are a part of a relatively new clinical toolset categorized by their clinical applications. The four main classes are molecular, physiologic, histologic and radiographic biomarkers. [3] All four types of biomarkers have a clinical role in narrowing or guiding treatment decisions and follow a sub-categorization of being either predictive, prognostic, or diagnostic.
Predictive
Predictive molecular, cellular, or imaging biomarkers that pass validation can serve as a method of predicting clinical outcomes. Predictive biomarkers are used to help optimize ideal treatments, and often indicate the likelihood of benefiting from a specific therapy. For example, molecular biomarkers situated at the interface of pathology-specific molecular process architecture and drug mechanism of action promise capturing aspects allowing assessment of an individual treatment response. [4] This offers a dual approach to both seeing trends in retrospective studies and using biomarkers to predict outcomes. For example, in metastatic colorectal cancer predictive biomarkers can serve as a way of evaluating and improving patient survival rates and in the individual case by case scenario, they can serve as a way of sparing patients from needless toxicity that arises from cancer treatment plans. [5]
Common examples of predictive biomarkers are genes such as ER, PR and HER2/neu in breast cancer, BCR-ABL fusion protein in chronic myeloid leukaemia, c-KIT mutations in GIST tumours and EGFR1 mutations in NSCLC. [6]
Diagnostic
Diagnostic biomarkers that meet a burden of proof can serve a role in narrowing down diagnosis. This can lead to diagnosis that are significantly more specific to individual patients.
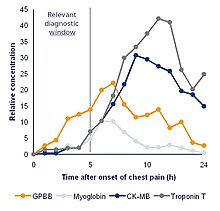
A biomarker can be a traceable substance that is introduced into an organism as a means to examine organ function or other aspects of health. [7] For example, rubidium chloride is used as a radioactive isotope to evaluate perfusion of heart muscle.[ citation needed ]
It can also be a substance whose detection indicates a particular disease state, for example, the presence of an antibody may indicate an infection. [7] More specifically, a biomarker indicates a change in expression or state of a protein that correlates with the risk or progression of a disease, or with the susceptibility of the disease to a given treatment. [7]
One example of a commonly used biomarker in medicine is prostate-specific antigen (PSA). This marker can be measured as a proxy of prostate size with rapid changes potentially indicating cancer. The most extreme case would be to detect mutant proteins as cancer specific biomarkers through selected reaction monitoring (SRM), since mutant proteins can only come from an existing tumor, thus providing ultimately the best specificity for medical purposes. [8]
An example is the traumatic brain injury (TBI) blood-based biomarker test consisted of measuring the levels of neuronal Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) and Glial fibrillary acidic protein (GFAP) to aid in the diagnosis of the presence of cranial lesion(s) among moderate to mild TBI patients that is(are) otherwise only diagnosable with the use of a CT scan of the head. [9]
Another example is KRAS, an oncogene that encodes a GTPase involved in several signal transduction pathways. Biomarkers for precision oncology are typically utilized in the molecular diagnostics of chronic myeloid leukemia, colon, breast, and lung cancer, and in melanoma. [10]
Digital
Digital biomarkers are a novel emerging field of biomarkers, mostly collected by smart biosensors. [11] So far, digital biomarkers have been focusing on monitoring vital parameters such as accelerometer data and heartrate [12] [13] but also speech. [14] Novel non-invasive, molecular digital biomarkers are increasingly available recorded by e.g. on-skin sweat analysis (internet-enabled Sudorology), which can be seen as next-generation digital biomarkers. [15] Collecting and tracking digital biomarkers have become more easily available with the advancement of smartphones and wearables. In Parkinson's disease (PD), for example, finger tapping a mobile phone via counting apps have been used as a method of (self-)evaluating bradykinesia and effectiveness of medication. [16] Digital biomarkers can be easily shared with the responsible physician, and novel diagnostics approaches can be developed using artificial intelligence.[ citation needed ]
Digital biomarkers are currently being used in conjugation with artificial intelligence (A.I.) in order to recognize symptoms for mild cognitive impairment (MCI). [17] One major current use of digital biomarkers involves keeping track of regular brain activity. Specific neural indicators can be measured by devices to evaluate patients for any neuro abnormalities. The data collected can determine the patients disease probability or condition. [18] While patients carryout everyday tasks (IADL), computers are using machine learning to observe and detect any deviation from normal behavior. These markers are used as signs or indicators of cognitive decline. [17]
Prognostic
A prognostic biomarker provides information about the patients overall outcome, regardless of any treatment or therapeutic intervention. [6] One example of a prognostic biomarkers in clinical research, is the use of mutated PIK3CA in the study of metastatic breast cancer. As illustrated by the graph, the mutation is prognostic since its presence in the patient endure the same outcome regardless of the treatment method used. Women who had the PIK3CA mutation before treatment, had the lowest average survival rate. The decline in the groups containing the mutant occurred quicker and in a much steeper decline. The independent nature of the prognostic factor allows researcher to study the disease or condition in its natural state. This makes it easier to observe these abnormal biological processes and speculate on how to correct them. Prognostic factors are often used in combination with predictive variables in therapeutics studies, to examine how effective different treatments are in curing specific diseases or cancer. As opposed to predictive biomarkers, prognostic do not rely on any explanatory variables, thus allowing for independent examination of the underlying disease or condition. [19]





