
Pralatrexate, sold under the brand name Folotyn, is a medication used for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL).

Trichostatin A (TSA) is an organic compound that serves as an antifungal antibiotic and selectively inhibits the class I and II mammalian histone deacetylase (HDAC) families of enzymes, but not class III HDACs. However, there are recent reports of the interactions of this molecule with Sirt 6 protein. TSA inhibits the eukaryotic cell cycle during the beginning of the growth stage. TSA can be used to alter gene expression by interfering with the removal of acetyl groups from histones and therefore altering the ability of DNA transcription factors to access the DNA molecules inside chromatin. It is a member of a larger class of histone deacetylase inhibitors that have a broad spectrum of epigenetic activities. Thus, TSA has some potential as an anti-cancer drug. One suggested mechanism is that TSA promotes the expression of apoptosis-related genes, leading to cancerous cells surviving at lower rates, thus slowing the progression of cancer. Other mechanisms may include the activity of HDIs to induce cell differentiation, thus acting to "mature" some of the de-differentiated cells found in tumors. HDIs have multiple effects on non-histone effector molecules, so the anti-cancer mechanisms are truly not understood at this time.
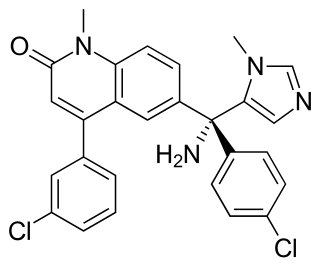
Tipifarnib is a farnesyltransferase inhibitor. Farnesyltransferase inhibitors block the activity of the farnesyltransferase enzyme by inhibiting prenylation of the CAAX tail motif, which ultimately prevents Ras from binding to the membrane, rendering it inactive.
Vorinostat (rINN) also known as suberanilohydroxamic acid is a member of a larger class of compounds that inhibit histone deacetylases (HDAC). Histone deacetylase inhibitors (HDI) have a broad spectrum of epigenetic activities.

TopoTarget was a Copenhagen-based biotechnology company focused on the discovery and development of drugs and therapies to treat cancer. In 2014, it merged with BioAlliance Pharma and is now part of Onxeo.

Panobinostat is a drug by Novartis for the treatment of various cancers. It is a hydroxamic acid and acts as a non-selective histone deacetylase inhibitor.
Spectrum Pharmaceuticals is an American biopharmaceutical company. The company is located in Irvine, California.

Romidepsin, also known as Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, now a part of Celgene.
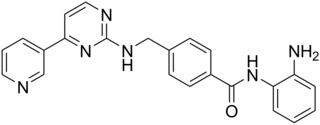
Mocetinostat (MGCD0103) is a benzamide histone deacetylase inhibitor undergoing clinical trials for treatment of various cancers including follicular lymphoma, Hodgkin's lymphoma and acute myelogenous leukemia.
Brentuximab vedotin is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL). It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium: The Takeda Oncology Company outside the US, and Seattle Genetics in the US.
A phosphoinositide 3-kinase inhibitor is a class of medical drug that functions by inhibiting one or more of the phosphoinositide 3-kinase enzymes, which are part of the PI3K/AKT/mTOR pathway, an important signalling pathway for many cellular functions such as growth control, metabolism and translation initiation. Within this pathway there are many components, inhibition of which may result in tumor suppression. These anti-cancer drugs are examples of targeted therapy.

Crizotinib is an anti-cancer drug acting as an ALK and ROS1 inhibitor, approved for treatment of some non-small cell lung carcinoma (NSCLC) in the US and some other countries, and undergoing clinical trials testing its safety and efficacy in anaplastic large cell lymphoma, neuroblastoma, and other advanced solid tumors in both adults and children.
ALK inhibitors are anti-cancer drugs that act on tumours with variations of anaplastic lymphoma kinase (ALK) such as an EML4-ALK translocation. They fall under the category of tyrosine kinase inhibitors, which work by inhibiting proteins involved in the abnormal growth of tumour cells. All the current approved ALK inhibitors function by binding to the ATP pocket of the abnormal ALK protein, blocking its access to energy and deactivating it. A majority of ALK-rearranged NSCLC harbour the EML4-ALK fusion, although as of 2020, over 92 fusion partners have been discovered in ALK+ NSCLC. For each fusion partner, there can be several fusion variants depending on the position the two genes were fused at, and this may have implications on the response of the tumour and prognosis of the patient.
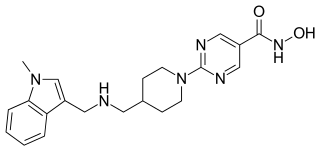
Quisinostat is an experimental drug candidate for the treatment of cancer. It is a "second generation" histone deacetylase inhibitor with antineoplastic activity. It is highly potent against class I and II HDACs.

Pracinostat (SB939) is an orally bioavailable, small-molecule histone deacetylase (HDAC) inhibitor based on hydroxamic acid with potential anti-tumor activity characterized by favorable physicochemical, pharmaceutical, and pharmacokinetic properties.
Resminostat is an orally bioavailable inhibitor of histone deacetylases (HDACs), of which inhibitors are antineoplastic agents.
In 2011, the German drug maker 4SC was granted orphan drug designation for resminostat by the US FDA for the treatment of hepatocellular carcinoma (HCC).

Copanlisib is a drug which is approved by US FDA for the treatment of adult patients experiencing relapsed follicular lymphoma who have received at least two prior systemic therapies.

Chidamide (Epidaza) is a histone deacetylase inhibitor (HDI) developed wholly in China. It was also known as HBI-8000. It is a benzamide HDI and inhibits Class I HDAC1, HDAC2, HDAC3, as well as Class IIb HDAC10.
Entrectinib, sold under the brand name Rozlytrek, is an anti-cancer medication used to treat ROS1-positive non-small cell lung cancer and NTRK fusion-positive solid tumors. It is a selective tyrosine kinase inhibitor (TKI), of the tropomyosin receptor kinases (TRK) A, B and C, C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK).

Lorlatinib, sold under the brand name Lorbrena in the United States, Canada, and Japan, and Lorviqua in the European Union, is an anti-cancer drug developed by Pfizer. It is an orally administered inhibitor of ALK and ROS1, two enzymes that play a role in the development of cancer.















