Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent or it may aim to prolong life or to reduce symptoms. Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called medical oncology.
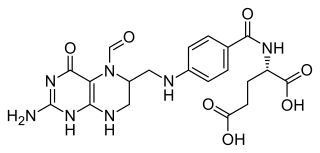
Folinic acid, also known as leucovorin, is a medication used to decrease the toxic effects of methotrexate and pyrimethamine. It is also used in combination with 5-fluorouracil to treat colorectal cancer and pancreatic cancer, may be used to treat folate deficiency that results in anemia, and methanol poisoning. It is taken by mouth, injection into a muscle, or injection into a vein.
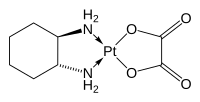
Carboplatin, sold under the brand name Paraplatin among others, is a chemotherapy medication used to treat a number of forms of cancer. This includes ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma. It is used by injection into a vein.
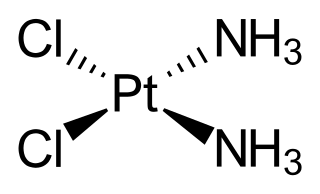
Cisplatin is a chemical compound with formula cis-[Pt(NH3)2Cl2]. It is a coordination complex of platinum that is used as a chemotherapy medication used to treat a number of cancers. These include testicular cancer, ovarian cancer, cervical cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelioma, brain tumors and neuroblastoma. It is given by injection into a vein.

Pemetrexed, sold under the brand name Alimta among others, is a chemotherapy medication for the treatment of pleural mesothelioma and non-small cell lung cancer (NSCLC)..

Cetuximab, sold under the brand name Erbitux, is an epidermal growth factor receptor (EGFR) inhibitor medication used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.
FOLFOX is a chemotherapy regimen for treatment of colorectal cancer, made up of the drugs folinic acid, fluorouracil, and oxaliplatin.

Irinotecan, sold under the brand name Camptosar among others, is an anti-cancer medication used to treat colon cancer and small cell lung cancer. For colon cancer it is used either alone or with fluorouracil. For small cell lung cancer it is used with cisplatin. It is given intravenously.

Floxuridine is an oncology drug that belongs to the class known as antimetabolites. Specifically, floxuridine is a pyrimidine analog, classified as a deoxyuridine. The drug is usually administered via an artery, and most often used in the treatment of colorectal cancer. The quality of life and survival rates of individuals that receive continuous hepatic artery infusion of floxuridine for colorectal cancer metastases is significantly higher than control groups. Floxuridine can also be prescribed for the treatment of kidney and stomach cancers. In vitro uses of floxuridine include 5-minute treatments of fluorouracil, floxuridine, and mitomycin to increase cell proliferation in Tenon's capsule fibroblasts.

Altretamine, also called hexamethylmelamine, is an antineoplastic agent. It was approved by the U.S. FDA in 1990.
FOLFIRI is a chemotherapy regimen for treatment of colorectal cancer. It is made up of the following drugs:
Triplatin tetranitrate is a platinum-based cytotoxic drug that underwent clinical trials for the treatment of human cancer. The drug acts by forming adducts with cellular DNA, preventing DNA transcription and replication, thereby inducing apoptosis. Other platinum-containing anticancer drugs include cisplatin, carboplatin, and oxaliplatin.
IFL is a chemotherapy regimen for treatment of certain cancers, consisting of concurrent treatment with irinotecan, leucovorin, and fluorouracil.
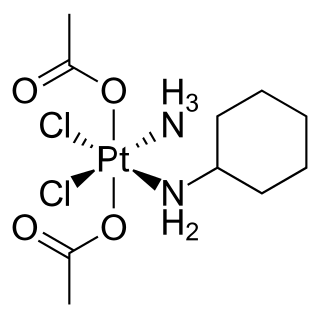
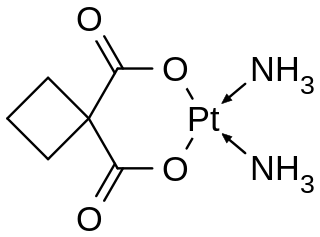
Satraplatin is a platinum-based antineoplastic agent that was under investigation as a treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues—cisplatin, carboplatin, and oxaliplatin—must be given intravenously.
Aflibercept, sold under the brand names Eylea among others, is a medication used to treat wet macular degeneration and metastatic colorectal cancer. It was developed by Regeneron Pharmaceuticals and is approved in the United States and the European Union.
Platinum-based antineoplastic drugs are chemotherapeutic agents used to treat cancer. Their active moieties are coordination complexes of platinum. These drugs are used to treat almost half of people receiving chemotherapy for cancer. In this form of chemotherapy, commonly used drugs include cisplatin, oxaliplatin, and carboplatin, but several have been proposed or are under development. Addition of platinum-based chemotherapy drugs to chemoradiation in women with early cervical cancer seems to improve survival and reduce risk of recurrence.
FOLFIRINOX is a chemotherapy regimen for treatment of advanced pancreatic cancer. It is made up of the following four drugs:
Tegafur/gimeracil/oteracil, sold under the brand name Teysuno among others is a fixed-dose combination medication used for the treatment of advanced gastric cancer when used in combination with cisplatin, and also for the treatment of head and neck cancer, colorectal cancer, non–small-cell lung, breast, pancreatic, and biliary tract cancers.
FOLFOXIRI is a chemotherapy regimen for the treatment of advanced colorectal cancer. The role of FOLFOXIRI in colorectal cancer has been reviewed.

Lobaplatin is a platinum-based antineoplastic metallodrug approved exclusively in China for the treatment of small cell lung cancer, inoperable metastatic breast cancer and chronic myelogenous leukaemia. The drug is a third-generation analogue of cisplatin, the first globally approved and widely used platinum-based anticancer drug.