
Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent or it may aim to prolong life or to reduce symptoms. Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called medical oncology.
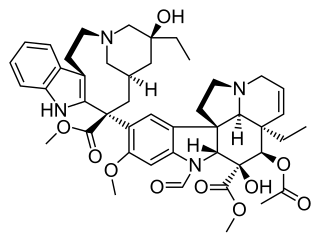
Vincristine, also known as leurocristine and marketed under the brand name Oncovin among others, is a chemotherapy medication used to treat a number of types of cancer. This includes acute lymphocytic leukemia, acute myeloid leukemia, Hodgkin's disease, neuroblastoma, and small cell lung cancer among others. It is given intravenously.
Harmine is a beta-carboline and a harmala alkaloid. It occurs in a number of different plants, most notably the Syrian rue and Banisteriopsis caapi. Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamines, making it a Reversible inhibitor of monoamine oxidase A (RIMA). Harmine does not inhibit MAO-B. Harmine is also known as banisterin, banisterine, telopathin, telepathine, leucoharmine and yagin, yageine.

Noscapine is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae. It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects.
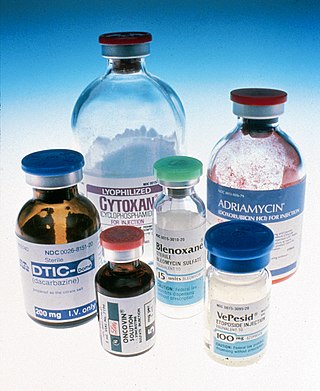
The era of cancer chemotherapy began in the 1940s with the first use of nitrogen mustards and folic acid antagonist drugs. The targeted therapy revolution has arrived, but many of the principles and limitations of chemotherapy discovered by the early researchers still apply.

Vinorelbine (NVB), sold under the brand name Navelbine among others, is a chemotherapy medication used to treat a number of types of cancer. This includes breast cancer and non-small cell lung cancer. It is given by injection into a vein or by mouth.

Vinblastine (VBL), sold under the brand name Velban among others, is a chemotherapy medication, typically used with other medications, to treat a number of types of cancer. This includes Hodgkin's lymphoma, non-small cell lung cancer, bladder cancer, brain cancer, melanoma, and testicular cancer. It is given by injection into a vein.

Taxanes are a class of diterpenes. They were originally identified from plants of the genus Taxus (yews), and feature a taxadiene core. Paclitaxel (Taxol) and docetaxel (Taxotere) are widely used as chemotherapy agents. Cabazitaxel was FDA approved to treat hormone-refractory prostate cancer.

Catharanthus roseus, commonly known as bright eyes, Cape periwinkle, graveyard plant, Madagascar periwinkle, old maid, pink periwinkle, rose periwinkle, is a perennial species of flowering plant in the family Apocynaceae. It is native and endemic to Madagascar, but grown elsewhere as an ornamental and medicinal plant. It is a source of the drugs vincristine and vinblastine, used to treat cancer. It was formerly included in the genus Vinca as Vinca rosea.

Cyclopamine (11-deoxojervine) is a naturally occurring chemical that belongs in the family of steroidal alkaloids. It is a teratogen isolated from the corn lily that causes fatal birth defects. It prevents the embryonic brain from separating into two lobes, which in turn causes the development of a single eye (cyclopia). The chemical was named after this effect, as it was originally noted by Idaho lamb farmers who contacted the US Department of Agriculture after their herds gave birth to cycloptic lambs in 1957. It then took more than a decade to identify the corn lily as the culprit. Later work suggested that different rain patterns caused the sheep to graze differently, impacting the amount of corn lily ingested by pregnant sheep. The poison interrupts the sonic hedgehog signaling pathway during development, thus causing birth defects.
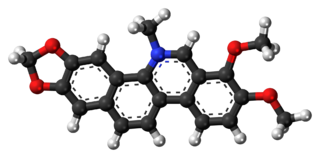
Chelerythrine is a benzophenanthridine alkaloid present in the plant Chelidonium majus. It is a potent, selective, and cell-permeable protein kinase C inhibitor in vitro. And an efficacious antagonist of G-protein-coupled CB1 receptors. This molecule also exhibits anticancer qualities and it has served as a base for many potential novel drugs against cancer. Structurally, this molecule has two distinct conformations, one being a positively charged iminium form, and the other being an uncharged form, a pseudo-base.

Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.
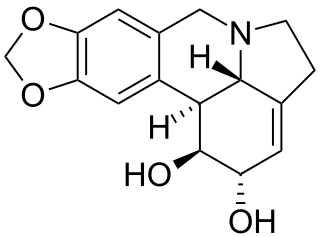
Lycorine is a toxic crystalline alkaloid found in various Amaryllidaceae species, such as the cultivated bush lily, surprise lilies (Lycoris), and daffodils (Narcissus). It may be highly poisonous, or even lethal, when ingested in certain quantities. Regardless, it is sometimes used medicinally, a reason why some groups may harvest the very popular Clivia miniata.

A mitotic inhibitor is a drug that inhibits mitosis, or cell division. These drugs disrupt microtubules, which are structures that pull the chromosomes apart when a cell divides. Mitotic inhibitors are used in cancer treatment, because cancer cells are able to grow and eventually spread through the body (metastasize) through continuous mitotic division. Thus, cancer cells are more sensitive to inhibition of mitosis than normal cells. Mitotic inhibitors are also used in cytogenetics, where they stop cell division at a stage where chromosomes can be easily examined.
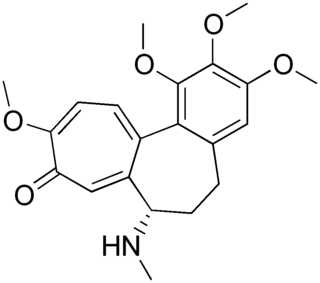
Demecolcine is a drug used in chemotherapy. It is closely related to the natural alkaloid colchicine with the replacement of the acetyl group on the amino moiety with methyl, but it is less toxic. It depolymerises microtubules and limits microtubule formation, thus arresting cells in metaphase and allowing cell harvest and karyotyping to be performed.

Dauricine is a plant metabolite, chemically classified as a phenol, an aromatic ether, and an isoquinoline alkaloid. It has been isolated from the Asian vine Menispermum dauricum, commonly known as Asian moonseed, and the North American vine Menispermum canadense, commonly known as Canadian moonseed. Scientists Tetsuji Kametani and Keiichiro Fukumoto of Japan are credited with being the first to synthesize dauricine in 1964, using both the Arndt-Eistert reaction and Bischler-Napieralski reaction to do so. Dauricine has been studied in vitro for its potential to inhibit cancer cell growth and to block cardiac transmembrane Na+, K+, and Ca2+ ion currents.

Mitotic Catastrophe has been defined as either a cellular mechanism to prevent potentially cancerous cells from proliferating or as a mode of cellular death that occurs following improper cell cycle progression or entrance. Mitotic catastrophe can be induced by prolonged activation of the spindle assembly checkpoint, errors in mitosis, or DNA damage and functioned to prevent genomic instability. It is a mechanism that is being researched as a potential therapeutic target in cancers, and numerous approved therapeutics induce mitotic catastrophe.

Volasertib is an experimental small molecule inhibitor of the PLK1 protein being developed by Boehringer Ingelheim for use as an anti-cancer agent. Volasertib is the second in a novel class of drugs called dihydropteridinone derivatives.
Tubulin inhibitors are chemotherapy drugs that interfere directly with the tubulin system, which is in contrast to those chemotherapy drugs acting on DNA. Microtubules play an important role in eukaryotic cells. Alpha- and beta-tubulin, the main components of microtubules, have gained considerable interest because of their function and biophysical properties and has become the subject of intense study. The addition of tubulin ligands can affect microtubule stability and function, including mitosis, cell motion and intracellular organelle transport. Tubulin binding molecules have generated significant interest after the introduction of the taxanes into clinical oncology and the general use of the vinca alkaloids. These compounds inhibit cell mitosis by binding to the protein tubulin in the mitotic spindle and preventing polymerization or depolymerization into the microtubules. This mode of action is also shared with another natural agent called colchicine.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.

















