
A myelodysplastic syndrome (MDS) is one of a group of cancers in which immature blood cells in the bone marrow do not mature, and as a result, do not develop into healthy blood cells. Early on, no symptoms typically are seen. Later, symptoms may include fatigue, shortness of breath, bleeding disorders, anemia, or frequent infections. Some types may develop into acute myeloid leukemia.

Bone marrow is a semi-solid tissue found within the spongy portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production. It is composed of hematopoietic cells, marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis. Bone marrow comprises approximately 5% of total body mass in healthy adult humans, such that a man weighing 73 kg (161 lbs) will have around 3.7 kg (8 lbs) of bone marrow.

Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes. Early on, there are typically no symptoms. Later, non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur. Enlargement of the spleen and low red blood cells (anemia) may also occur. It typically worsens gradually over years.
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood in order to replicate inside of a patient and to produce additional normal blood cells. It may be autologous, allogeneic or syngeneic.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the very first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.

Cell therapy is a therapy in which viable cells are injected, grafted or implanted into a patient in order to effectuate a medicinal effect, for example, by transplanting T-cells capable of fighting cancer cells via cell-mediated immunity in the course of immunotherapy, or grafting stem cells to regenerate diseased tissues.

Melphalan, sold under the brand name Alkeran among others, is a chemotherapy medication used to treat multiple myeloma; malignant lymphoma; lymphoblastic and myeloblastic leukemia; childhood neuroblastoma; ovarian cancer; mammary adenocarcinoma; and uveal melanoma. It is taken by mouth or by injection into a vein.
Total body irradiation (TBI) is a form of radiotherapy used primarily as part of the preparative regimen for haematopoietic stem cell transplantation. As the name implies, TBI involves irradiation of the entire body, though in modern practice the lungs are often partially shielded to lower the risk of radiation-induced lung injury. Total body irradiation in the setting of bone marrow transplantation serves to destroy or suppress the recipient's immune system, preventing immunologic rejection of transplanted donor bone marrow or blood stem cells. Additionally, high doses of total body irradiation can eradicate residual cancer cells in the transplant recipient, increasing the likelihood that the transplant will be successful.

Thiotepa (INN), sold under the brand name Tepadina, is a medication used to treat cancer.
Defibrotide, sold under the brand name Defitelio, is a mixture of single-stranded oligonucleotides that is purified from the intestinal mucosa of pigs. It is used to treat veno-occlusive disease of the liver of people having had a bone marrow transplant, with different limitations in the US and the European Union. It works by protecting the cells lining blood vessels in the liver and preventing blood clotting; the way it does this is not well understood.
Hepatosplenic T-cell lymphoma is a rare form of lymphoma that is generally incurable, except in the case of an allogeneic stem cell transplant. It is a systemic neoplasm comprising medium-sized cytotoxic T-cells that show significant sinusoidal infiltration in the liver, spleen, and bone marrow.
Juvenile myelomonocytic leukemia (JMML) is a rare form of chronic leukemia that affects children, commonly those aged four and younger. The name JMML now encompasses all diagnoses formerly referred to as juvenile chronic myeloid leukemia (JCML), chronic myelomonocytic leukemia of infancy, and infantile monosomy 7 syndrome. The average age of patients at diagnosis is two (2) years old. The World Health Organization has included JMML as a subcategory of myelodysplastic and myeloproliferative disorders.
Peripheral blood stem cell transplantation (PBSCT), also called "Peripheral stem cell support", is a method of replacing blood-forming stem cells. Stem cells can be destroyed through cancer treatments such as chemotherapy or radiation, as well as any blood-related diseases, such as leukemia, lymphoma, neuroblastoma and multiple myeloma. PBSCT is now a much more common procedure than its bone marrow harvest equivalent due to the ease and less invasive nature of the procedure. Studies suggest that PBSCT has a better outcome in terms of the number of hematopoietic stem cell yield.

Maribavir, sold under the brand name Livtencity, is an antiviral medication that is used to treat post-transplant cytomegalovirus (CMV). Maribavir is a cytomegalovirus pUL97 kinase inhibitor that works by preventing the activity of human cytomegalovirus enzyme pUL97, thus blocking virus replication.
Graft-versus-tumor effect (GvT) appears after allogeneic hematopoietic stem cell transplantation (HSCT). The graft contains donor T cells that can be beneficial for the recipient by eliminating residual malignant cells. GvT might develop after recognizing tumor-specific or recipient-specific alloantigens. It could lead to remission or immune control of hematologic malignancies. This effect applies in myeloma and lymphoid leukemias, lymphoma, multiple myeloma and possibly breast cancer. It is closely linked with graft-versus-host disease (GvHD), as the underlying principle of alloimmunity is the same. CD4+CD25+ regulatory T cells (Treg) can be used to suppress GvHD without loss of beneficial GvT effect. The biology of GvT response is still not fully understood but it is probable that the reaction with polymorphic minor histocompatibility antigens expressed either specifically on hematopoietic cells or more widely on a number of tissue cells or tumor-associated antigens is involved. This response is mediated largely by cytotoxic T lymphocytes (CTL) but it can be employed by natural killers as separate effectors, particularly in T-cell-depleted HLA-haploidentical HSCT.
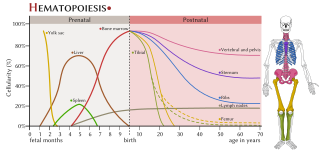
The haematopoietic system is the system in the body involved in the creation of the cells of blood.
FLAG is a chemotherapy regimen used for relapsed and refractory acute myeloid leukemia (AML). The acronym incorporates the three primary ingredients of the regimen:
- Fludarabine: an antimetabolite that, while not active toward AML, increases formation of an active cytarabine metabolite, ara-CTP, in AML cells;
- Arabinofuranosyl cytidine : an antimetabolite that has been proven to be the most active toward AML among various cytotoxic drugs in single-drug trials; and
- Granulocyte colony-stimulating factor (G-CSF): a glycoprotein that shortens the duration and severity of neutropenia.
Guo Mei is a hematologist and associate director of 307th Hospital of Chinese People’s Liberation Army and deputy director of Radiation Research Institute.

Shimon Slavin is an Israeli professor of medicine. Slavin pioneered the use of immunotherapy mediated by allogeneic donor lymphocytes and innovative methods for stem cell transplantation for the cure of hematological malignancies and solid tumors, and using hematopoietic stem cells for induction of transplantation tolerance to bone marrow and donor allografts.













