Cholangiocarcinoma, also known as bile duct cancer, is a type of cancer that forms in the bile ducts. Symptoms of cholangiocarcinoma may include abdominal pain, yellowish skin, weight loss, generalized itching, and fever. Light colored stool or dark urine may also occur. Other biliary tract cancers include gallbladder cancer and cancer of the ampulla of Vater.
Fluorouracil, sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts.
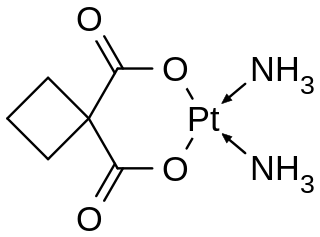
Carboplatin, sold under the brand name Paraplatin among others, is a chemotherapy medication used to treat a number of forms of cancer. This includes ovarian cancer, lung cancer, head and neck cancer, brain cancer, and neuroblastoma. It is used by injection into a vein.
An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid; thus, competitive inhibition can occur, and the presence of antimetabolites can have toxic effects on cells, such as halting cell growth and cell division, so these compounds are used in chemotherapy for cancer.
The era of cancer chemotherapy began in the 1940s with the first use of nitrogen mustards and folic acid antagonist drugs. The targeted therapy revolution has arrived, but many of the principles and limitations of chemotherapy discovered by the early researchers still apply.

Oxaliplatin, sold under the brand name Eloxatin among others, is a cancer medication used to treat colorectal cancer. It is given by injection into a vein.

Epirubicin is an anthracycline drug used for chemotherapy. It can be used in combination with other medications to treat breast cancer in patients who have had surgery to remove the tumor. It is marketed by Pfizer under the trade name Ellence in the US and Pharmorubicin or Epirubicin Ebewe elsewhere.
Adjuvant therapy, also known as adjunct therapy, adjuvant care, or augmentation therapy, is a therapy that is given in addition to the primary or initial therapy to maximize its effectiveness. The surgeries and complex treatment regimens used in cancer therapy have led the term to be used mainly to describe adjuvant cancer treatments. An example of such adjuvant therapy is the additional treatment usually given after surgery where all detectable disease has been removed, but where there remains a statistical risk of relapse due to the presence of undetected disease. If known disease is left behind following surgery, then further treatment is not technically adjuvant.
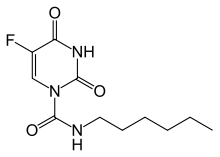
Tegafur/uracil is a chemotherapy drug combination used in the treatment of cancer, primarily bowel cancer. It is also called UFT or UFUR.

The ASAH1 gene encodes in humans the acid ceramidase enzyme.

Fibroblast growth factor 19 is a protein that in humans is encoded by the FGF19 gene. It functions as a hormone, regulating bile acid synthesis, with effects on glucose and lipid metabolism. Reduced synthesis, and blood levels, may be a factor in chronic bile acid diarrhea and in certain metabolic disorders.
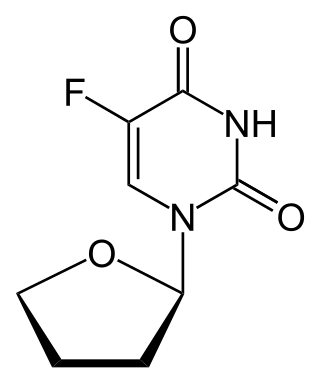
Tegafur is a chemotherapeutic prodrug of 5-fluorouracil (5-FU) used in the treatment of cancers. It is a component of the combination drug tegafur/uracil. When metabolised, it becomes 5-FU.

Polysaccharide-K is a protein-bound polysaccharide isolated from the mycelium of Trametes versicolor.
Wafik El-Deiry is an American physician and cancer researcher who is the Associate Dean for Oncologic Sciences at the Warren Alpert Medical School, Brown University, Director of the Cancer Center at Brown University, and the Director of the Joint Program in Cancer Biology at Brown University and its affiliated hospitals. He was previously deputy director of Translational Research at Fox Chase Cancer Center, where he was also co-Leader of the Molecular Therapeutics Program.
Platinum-based antineoplastic drugs are chemotherapeutic agents used to treat cancer. Their active moieties are coordination complexes of platinum. These drugs are used to treat almost half of people receiving chemotherapy for cancer. In this form of chemotherapy, commonly used drugs include cisplatin, oxaliplatin, and carboplatin, but several have been proposed or are under development. Addition of platinum-based chemotherapy drugs to chemoradiation in women with early cervical cancer seems to improve survival and reduce risk of recurrence.
Antineoplastic resistance, often used interchangeably with chemotherapy resistance, is the resistance of neoplastic (cancerous) cells, or the ability of cancer cells to survive and grow despite anti-cancer therapies. In some cases, cancers can evolve resistance to multiple drugs, called multiple drug resistance.
FOLFIRINOX is a chemotherapy regimen for treatment of advanced pancreatic cancer. It is made up of the following four drugs:
FOLFOXIRI is a chemotherapy regimen for the treatment of advanced colorectal cancer. The role of FOLFOXIRI in colorectal cancer has been reviewed.
Metronomic therapy is a new type of chemotherapy in which anti-cancer drugs are administered in a lower dose than the maximum tolerated dose repetitively over a long period to treat cancers with fewer side effects. Metronomic therapy is shown to affect both tumor microenvironment and tumor cells to achieve its therapeutic effects. Metronomic therapy is also cost-effective as a lower dose is used compared to conventional chemotherapy. The use of metronomic therapy has been extensively investigated and can be advantageous in selected group of patients. Yet, more clinical trials are necessary to generalize the method.

BOLD-100, or sodium trans-[tetrachlorobis (1H-indazole)ruthenate(III)], is a ruthenium-based anti-cancer therapeutic in clinical development. As of November 2021, BOLD-100 was being tested in a Phase 1b clinical trial in patients with advanced gastrointestinal cancers in combination with the chemotherapy regimen FOLFOX. BOLD-100 is being developed by Bold Therapeutics Inc.