Substances, mixtures and exposure circumstances in this list have been classified by the International Agency for Research on Cancer (IARC) as group 3: The agent is not classifiable as to its carcinogenicity to humans. This category is used most commonly for agents, mixtures and exposure circumstances for which the evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.

Triose-phosphate isomerase is an enzyme that catalyzes the reversible interconversion of the triose phosphate isomers dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate.
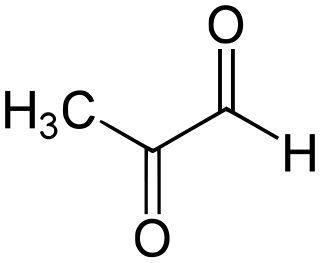
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase.
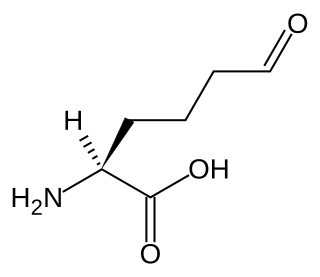
Allysine is a derivative of lysine, used in the production of elastin and collagen. It is produced by the actions of the enzyme lysyl oxidase in the extracellular matrix and is essential in the crosslink formation that stabilizes collagen and elastin.
The methylglyoxal pathway is an offshoot of glycolysis found in some prokaryotes, which converts glucose into methylglyoxal and then into pyruvate. However unlike glycolysis the methylglyoxal pathway does not produce adenosine triphosphate, ATP. The pathway is named after the substrate methylglyoxal which has three carbons and two carbonyl groups located on the 1st carbon and one on the 2nd carbon. Methylglyoxal is, however, a reactive aldehyde that is very toxic to cells, it can inhibit growth in E. coli at milimolar concentrations. The excessive intake of glucose by a cell is the most important process for the activation of the methylglyoxal pathway.
In enzymology, a methylglyoxal reductase (NADH-dependent) (EC 1.1.1.78) is an enzyme that catalyzes the chemical reaction
In enzymology, a methylglyoxal reductase (NADPH-dependent) (EC 1.1.1.283) is an enzyme that catalyzes the chemical reaction
In enzymology, a D-lactate dehydrogenase (cytochrome) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-oxoaldehyde dehydrogenase (NAD+) (EC 1.2.1.23) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-oxoaldehyde dehydrogenase (NADP+) (EC 1.2.1.49) is an enzyme that catalyzes the chemical reaction

Lactaldehyde is an intermediate in the methylglyoxal metabolic pathway. Methylglyoxal is converted to D-lactaldehyde by glycerol dehydrogenase (gldA). Lactaldehyde is then oxidized to lactic acid by aldehyde dehydrogenase.
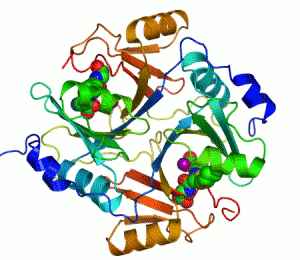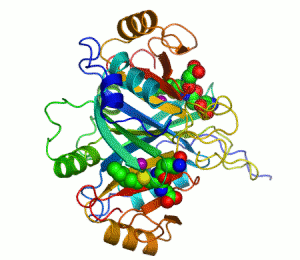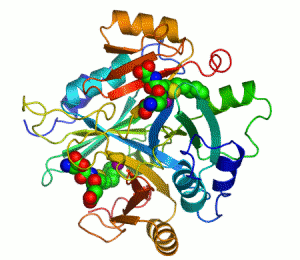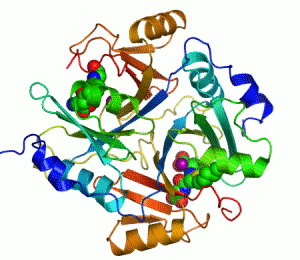
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal.
The enzyme methylglyoxal synthase catalyzes the chemical reaction
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism. This system has been studied in both bacteria and eukaryotes. This detoxification is accomplished by the sequential action of two thiol-dependent enzymes; firstly glyoxalase І, which catalyzes the isomerization of the spontaneously formed hemithioacetal adduct between glutathione and 2-oxoaldehydes into S-2-hydroxyacylglutathione. Secondly, glyoxalase ІІ hydrolyses these thiolesters and in the case of methylglyoxal catabolism, produces D-lactate and GSH from S-D-lactoyl-glutathione.

Semapimod (INN), formerly known as CNI-1493, is an investigational new drug which has anti-inflammatory, anti-cytokine, immunomodulatory, antiviral and antimalarial properties.
6-deoxy-5-ketofructose 1-phosphate synthase is an enzyme with systematic name 2-oxopropanal:D-fructose 1,6-bisphosphate glycerone-phosphotransferase. This enzyme catalyses the following chemical reaction
D-lactate dehydratase (EC 4.2.1.130, glyoxylase III) is an enzyme with systematic name (R)-lactate hydro-lyase. This enzyme catalyses the following chemical reaction
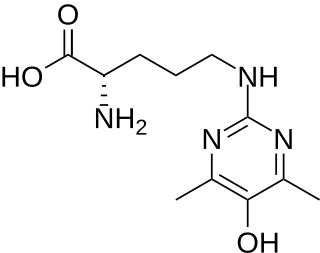
Argpyrimidine is an organic compound with the chemical formula C11H18N4O3. It is an advanced glycation end-product formed from arginine and methylglyoxal through the Maillard reaction. Argpyrimidine has been studied for its food chemistry purposes and its potential involvement in aging diseases and Diabetes Mellius.
Manju Ray was an Indian scientist specializing in Molecular Enzymology and Cancer Biochemistry. Her research has contributed significantly to the development of anticancer drugs and understanding the differentiation process of cells. Her interests include tumor biochemistry and molecular enzymology. She was awarded the Shanti Swarup Bhatnagar Prize for Science and Technology in the year 1989, being only the second woman to receive this award in the category 'Biological Sciences'.
Lethal synthesis, or suicide metabolism, is the biosynthesis of a toxin from a precursor which is not itself toxic, such as the synthesis of fluorocitrate from fluoroacetate or the synthesis of methylglyoxal from glycerol.