Related Research Articles
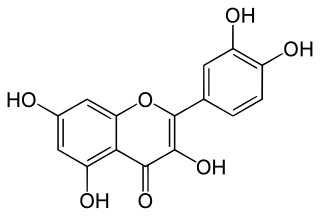
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
Parietaria officinalis, the eastern pellitory-of-the-wall, also known as upright pellitory and lichwort, is a plant of the nettle family. Its leaves, however, are non-stinging. The plant grows on rubbish and on walls, hence the name.

Malvidin is an O-methylated anthocyanidin, the 3',5'-methoxy derivative of delphinidin. As a primary plant pigment, its glycosides are highly abundant in nature.
In enzymology, an isoflavone 7-O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a myricetin O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a secologanin synthase (EC 1.14.19.62, was wrongly classified as EC 1.3.3.9 in the past) is an enzyme that catalyzes the chemical reaction

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Rosinidin is an O-methylated anthocyanidin derived from Cyanidin. It is a pigment found in the flowers of Catharanthus roseus and, in lower concentration, in Primula rosea.
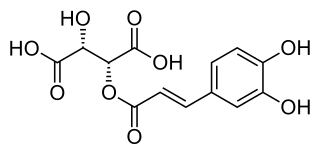
Caftaric acid is a non-flavonoid phenolic compound.

Irigenin is an O-methylated isoflavone, a type of flavonoid. It can be isolated from the rhizomes of the leopard lily, and Iris kemaonensis.

Prenylated flavonoids or prenylflavonoids are a sub-class of flavonoids. They are widely distributed throughout the plant kingdom. Some are known to have phytoestrogenic or antioxidant properties. They are given in the list of adaptogens in herbalism. Chemically they have a prenyl group attached to their flavonoid backbone. It is usually assumed that the addition of hydrophobic prenyl groups facilitate attachment to cell membranes. Prenylation may increase the potential activity of its original flavonoid.

Ayanin is an O-methylated flavonol, a type of flavonoid. It is the 3,7,4'-tri-O-methylated derivative of quercetin.

Ombuin is an O-methylated flavonol, a type of flavonoid. It is the 4',7-O-methyl derivative of quercetin.
An O-methyltransferase (OMT) is a type of methyltransferase enzyme transferring a methyl group on a molecule.

Syringetin is an O-methylated flavonol, a type of flavonoid. It is found in red grape, in Lysimachia congestiflora and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.

Calycosin is an O-methylated isoflavone. It can be isolated from Astragalus membranaceus Bge. var. mongholicus and Trifolium pratense L..

Mearnsetin is an O-methylated flavonol. It can be found in Eucalyptus globulus and in Elaeocarpus lanceofolius. The compound has antioxidative properties.
Flavonoid 4'-O-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:flavonoid 4'-O-methyltransferase. This enzyme catalyses the following chemical reaction

3-Hydroxy-16-methoxy-2,3-dihydrotabersonine is a terpene indole alkaloid produced by Catharanthus roseus. The metabolite is a substrate for 3-hydroxy-16-methoxy-2,3-dihydrotabersonine N-methyltransferase (NMT) which transfers a methyl group to the nitrogen of the indole ring forming desacetoxyvindoline. The enzyme catalyzing the formation of 3-hydroxy-16-methoxy-2,3-dihydrotabersonine from 16-methoxytabersonine is currently unknown, but is a result of hydration of the double bond connecting the 6 and 13 position carbons.

Pisatin (3-hydroxy-7-methoxy-4′,5′-methylenedioxy-chromanocoumarane) is the major phytoalexin made by the pea plant Pisum sativum. It was the first phytoalexin to be purified and chemically identified. The molecular formula is C17H14O6.
References
- ↑ Kim, Dae Hwan; Kim, Bong-Gyu; Lee, Youngshim; Ryu, Ji Young; Lim, Yoongho; Hur, Hor-Gil; Ahn, Joong-Hoon (2005). "Regiospecific methylation of naringenin to ponciretin by soybean O-methyltransferase expressed in Escherichia coli". Journal of Biotechnology. 119 (2): 155–62. doi:10.1016/j.jbiotec.2005.04.004. PMID 15961179.
- ↑ Schroder, G; Wehinger, E; Lukacin, R; Wellmann, F; Seefelder, W; Schwab, W; Schröder, J (2004). "Flavonoid methylation: a novel 4′-O-methyltransferase from Catharanthus roseus, and evidence that partially methylated flavanones are substrates of four different flavonoid dioxygenases". Phytochemistry. 65 (8): 1085–94. doi:10.1016/j.phytochem.2004.02.010. PMID 15110688.
- ↑ Kim, Bong-Gyu; Lee, Youngshim; Hur, Hor-Gil; Lim, Yoongho; Ahn, Joong-Hoon (2006). "Flavonoid 3′-O-methyltransferase from rice: CDNA cloning, characterization and functional expression". Phytochemistry. 67 (4): 387–94. doi:10.1016/j.phytochem.2005.11.022. PMID 16412485.
- ↑ Brunet, Gunter; Ibrahim, Ragai K. (1980). "O-methylation of flavonoids by cell-free extracts of calamondin orange". Phytochemistry. 19 (5): 741–6. doi:10.1016/0031-9422(80)85102-8.
- ↑ Harborne, J.B. (1967). "Comparative biochemistry of the flavonoids-IV.: Correlations between chemistry, pollen morphology and systematics in the family plumbaginaceae". Phytochemistry. 6 (10): 1415–28. doi:10.1016/S0031-9422(00)82884-8.