
Flunitrazepam, also known as Rohypnol among other names, is a benzodiazepine used to treat severe insomnia and assist with anesthesia. As with other hypnotics, flunitrazepam has been advised to be prescribed only for short-term use or by those with chronic insomnia on an occasional basis.
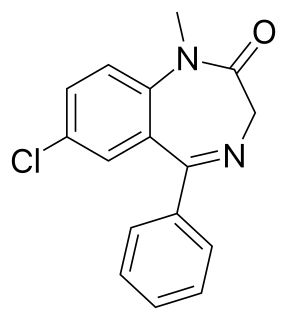
Diazepam, first marketed as Valium, is a medicine of the benzodiazepine family that acts as an anxiolytic. It is commonly used to treat a range of conditions, including anxiety, seizures, alcohol withdrawal syndrome, muscle spasms, insomnia, and restless legs syndrome. It may also be used to cause memory loss during certain medical procedures. It can be taken by mouth, inserted into the rectum, injected into muscle, injected into a vein or used as a nasal spray. When given into a vein, effects begin in one to five minutes and last up to an hour. By mouth, effects begin after 15 to 60 minutes.

Zopiclone, sold under the brand name Imovane among others, is a nonbenzodiazepine used to treat difficulty sleeping. Zopiclone is molecularly distinct from benzodiazepine drugs and is classed as a cyclopyrrolone. However, zopiclone increases the normal transmission of the neurotransmitter gamma-aminobutyric acid (GABA) in the central nervous system, via modulating GABAA receptors similarly to the way benzodiazepine drugs do.
Flurazepam is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties. It produces a metabolite with a long half-life, which may stay in the bloodstream for days. Flurazepam was patented in 1968 and came into medical use the same year. Flurazepam, developed by Roche Pharmaceuticals was one of the first benzo hypnotics to be marketed.

Quazepam, sold under brand name Doral among others, is a relatively long-acting benzodiazepine derivative drug developed by the Schering Corporation in the 1970s. Quazepam is used for the treatment of insomnia including sleep induction and sleep maintenance. Quazepam induces impairment of motor function and has relatively selective hypnotic and anticonvulsant properties with considerably less overdose potential than other benzodiazepines. Quazepam is an effective hypnotic which induces and maintains sleep without disruption of the sleep architecture.

Estazolam, sold under the brand name Prosom among others, is a tranquilizer medication of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties. Estazolam is an intermediate-acting oral benzodiazepine. It is used for short-term treatment of insomnia.

Clorazepate, sold under the brand name Tranxene among others, is a benzodiazepine medication. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. Clorazepate is an unusually long-lasting benzodiazepine and serves as a majoritive prodrug for the equally long-lasting desmethyldiazepam, which is rapidly produced as an active metabolite. Desmethyldiazepam is responsible for most of the therapeutic effects of clorazepate.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.
Fludiazepam, marketed under the brand name Erispan (エリスパン) is a potent benzodiazepine and 2ʹ-fluoro derivative of diazepam, originally developed by Hoffman-La Roche in the 1960s. It is marketed in Japan and Taiwan. It exerts its pharmacological properties via enhancement of GABAergic inhibition. Fludiazepam has 4 times more binding affinity for benzodiazepine receptors than diazepam. It possesses anxiolytic, anticonvulsant, sedative, hypnotic and skeletal muscle relaxant properties. Fludiazepam has been used recreationally.

Ethyl loflazepate is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses. Its elimination half-life is 51–103 hours. Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound. Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate. The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate. Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment. The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children. Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity. High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.

Clotiazepam is a thienodiazepine drug which is a benzodiazepine analog. The clotiazepam molecule differs from benzodiazepines in that the benzene ring has been replaced by a thiophene ring. It possesses anxiolytic, skeletal muscle relaxant, anticonvulsant, sedative properties. Stage 2 NREM sleep is significantly increased by clotiazepam.

Lormetazepam, sold under the brand name Noctamid among others, is a drug which is a short to intermediate acting 3-hydroxy benzodiazepine derivative and temazepam analogue. It possesses hypnotic, anxiolytic, anticonvulsant, sedative, and skeletal muscle relaxant properties.
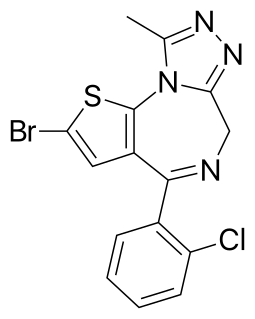
Brotizolam is a sedative-hypnotic thienotriazolodiazepine drug which is a benzodiazepine analog. It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties, and is considered to be similar in effect to other short-acting hypnotic benzodiazepines such as triazolam or midazolam. It is used in the short-term treatment of severe insomnia. Brotizolam is a highly potent and short-acting hypnotic, with a typical dose ranging from 0.125 to 0.25 milligrams, which is rapidly eliminated with an average half-life of 4.4 hours.

Mexazolam is a drug which is a benzodiazepine derivative. Mexazolam has been trialed for anxiety and was found to be effective in alleviating anxiety at one week follow-up. Mexazolam is metabolised via the CYP3A4 pathway. HMG-CoA reductase inhibitors including simvastatin, simvastatin acid, lovastatin, fluvastatin, atorvastatin and cerivastatin inhibit the metabolism of mexazolam, but not the HMG-CoA reductase inhibitor pravastatin. Its principal active metabolites are chlordesmethyldiazepam and chloroxazepam.
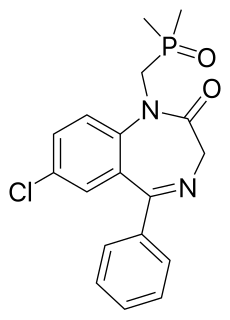
Fosazepam is a drug which is a benzodiazepine derivative; it is a water soluble derivative of diazepam. It has sedative and anxiolytic effects, and is a derivative of diazepam which has been substituted with a dimethylphosphoryl group to improve solubility in water.

Flutoprazepam (Restas) is a drug which is a benzodiazepine. It was patented in Japan by Sumitomo in 1972 and its medical use remains mostly confined to that country. Its muscle relaxant properties are approximately equivalent to those of diazepam - however, it has more powerful sedative, hypnotic, anxiolytic and anticonvulsant effects and is around four times more potent by weight compared to diazepam. It is longer acting than diazepam due to its long-acting active metabolites, which contribute significantly to its effects. Its principal active metabolite is n-desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam.

Rilmazafone is a water-soluble prodrug developed in Japan. Once metabolized, rilmazafone is converted into several benzodiazepine metabolites that have sedative and hypnotic effects. These metabolites induce impairment of motor function and have hypnotic properties.

Y-23684 is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
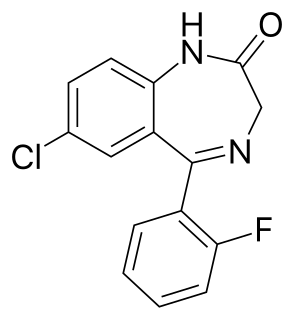
N-Desalkylflurazepam is a benzodiazepine analog and an active metabolite of several other benzodiazepine drugs including flurazepam, flutoprazepam, fludiazepam, midazolam, flutazolam, quazepam, and ethyl loflazepate. It is long-acting, prone to accumulation, and binds unselectively to the various benzodiazepine receptor subtypes. It has been sold as a designer drug from 2016 onward.
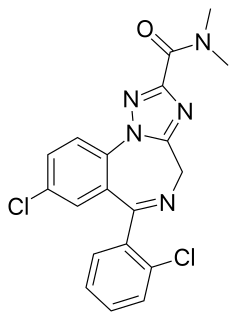
CCRIS-1930, is a benzodiazepine derivative which acts as a sedative and hypnotic drug, and the active metabolite of the drug Rilmazafone. It has never been developed for medical uses, yet the aforementioned prodrug is an approved medication in Japan.





















